![]() 7 Feb 2024
7 Feb 2024
The first commercial patient who underwent CAR T Cell Therapy was declared cancer-free.
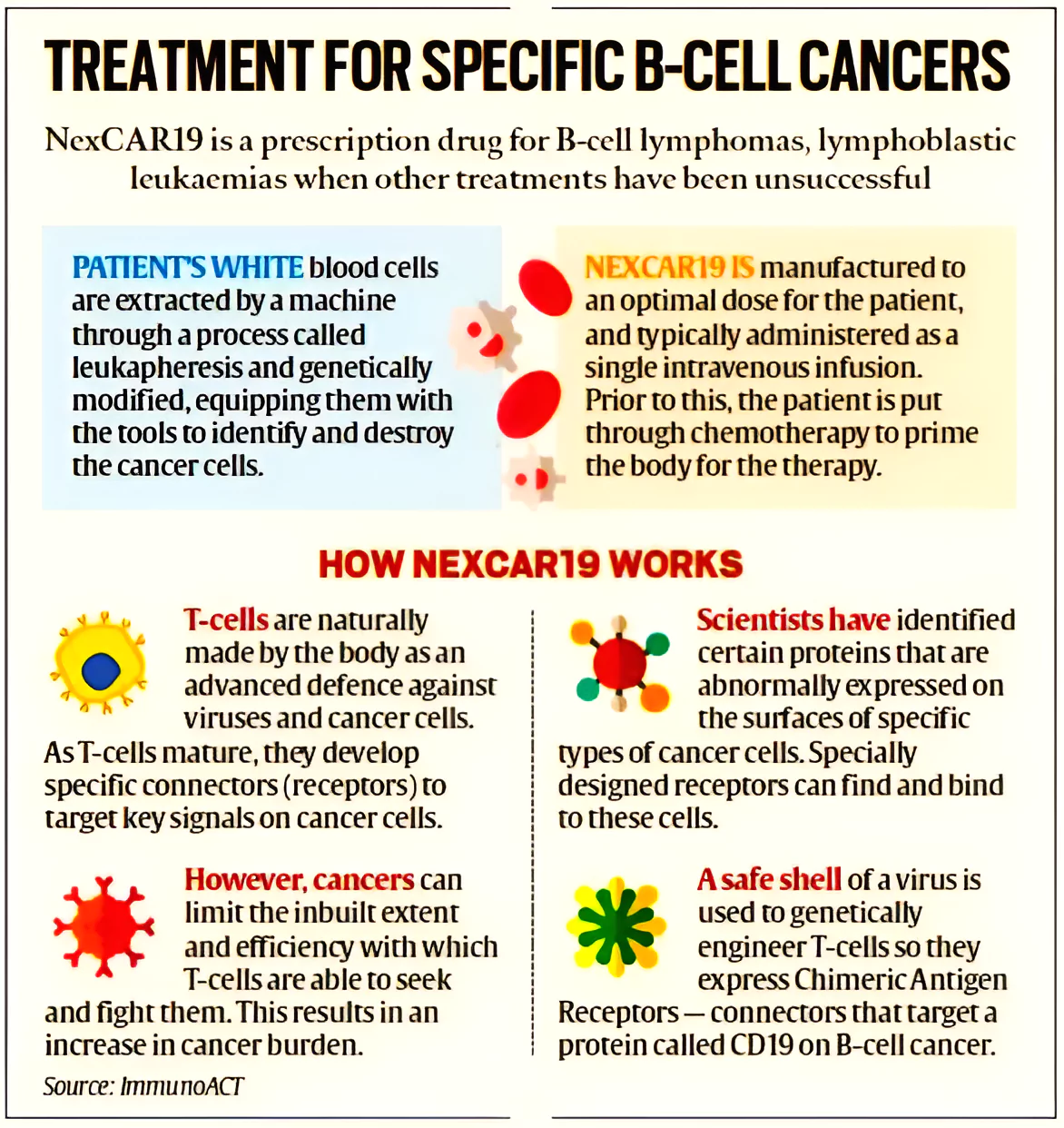
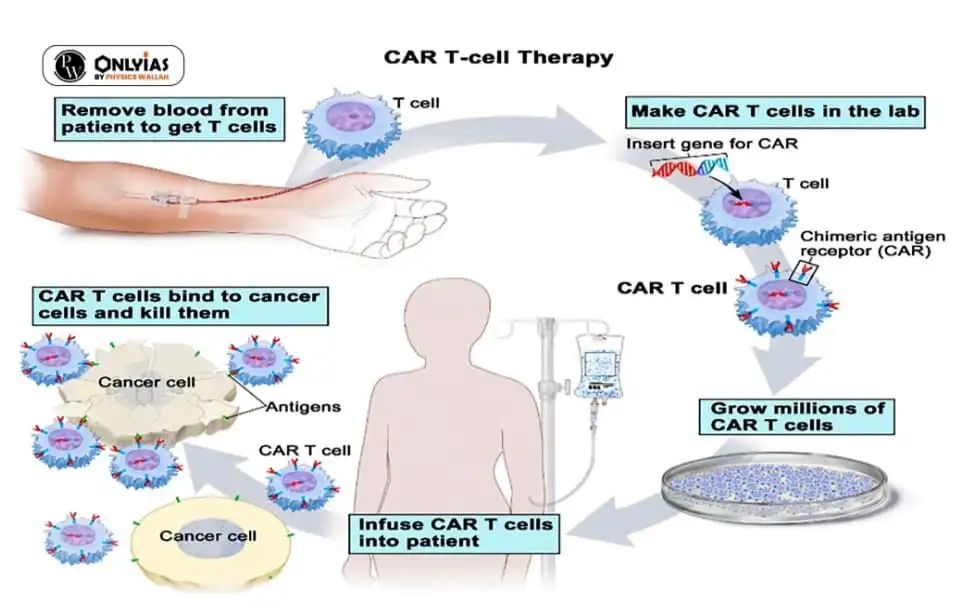 The genetically modified cells express chimeric antigen receptors (CARs) specific to cancer cells.
The genetically modified cells express chimeric antigen receptors (CARs) specific to cancer cells. News Source: Indian Express
| Must Read | |
| NCERT Notes For UPSC | UPSC Daily Current Affairs |
| UPSC Blogs | UPSC Daily Editorials |
| Daily Current Affairs Quiz | Daily Main Answer Writing |
| UPSC Mains Previous Year Papers | UPSC Test Series 2024 |
<div class="new-fform">
</div>