![]() 16 Dec 2023
16 Dec 2023
Salts are ionic compounds composed of positively charged ions (cations) and negatively charged ions (anions). Salts play vital roles in various biological and chemical processes, from maintaining electrolyte balance in the human body to participating in chemical reactions in industry and agriculture. Their diverse properties make them integral to numerous aspects of science and daily life.
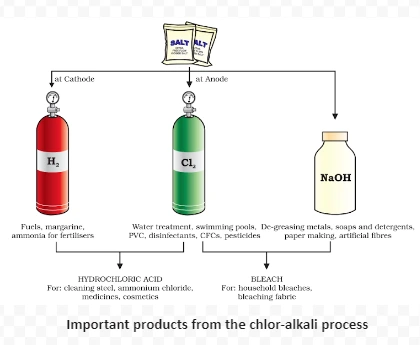
2NaCl(aq) + 2H2O(l) → 2NaOH(aq) + Cl2(g) + H2(g)
Ca(OH)2 + Cl2 → CaOCl2 + H2O
NaCl + H2O + CO2 + NH3 → NH4Cl (Ammonium chloride)+NaHCO3(Sodium Hydrogencarbonate)
2NaHCO3 → Na2CO3 (Sodium carbonate) + H2O + CO2
NaHCO3 + H+ (From any acid) → CO2 + H2O + Sodium salt of acid
Carbon dioxide produced during the reaction can cause bread or cake to rise making them soft and spongy.
Na2CO3 + 10 H2O → Na2CO3.10H2O
Chemical formula for hydrated copper sulphate is CuSO4.5H2O.
CaSO4. 1/2 H2O + 11/2 H2O → CaSO4.2H2O
<div class="new-fform">
</div>