Metals exhibit various distinctive properties, including conductivity, malleability, ductility, luster, and high melting points. They tend to form positive ions and participate in metallic bonding, contributing to their widespread use in construction, electronics, and manufacturing.
2Cu + O2 → 2CuO
(Copper) (Copper (II) oxide)
4Al + 3O2 → 2Al2O3
(Aluminium) (Aluminium oxide)
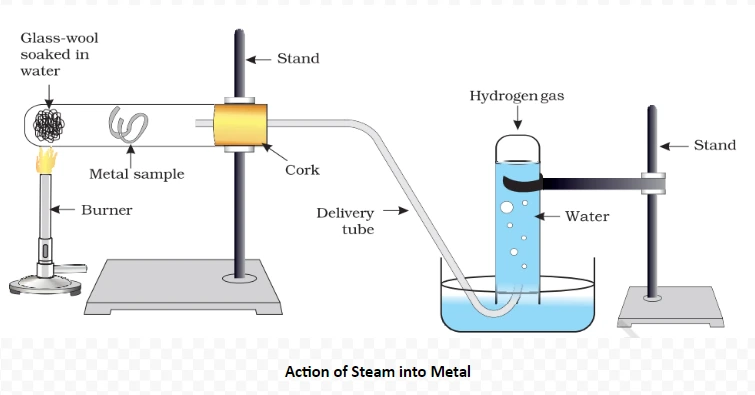
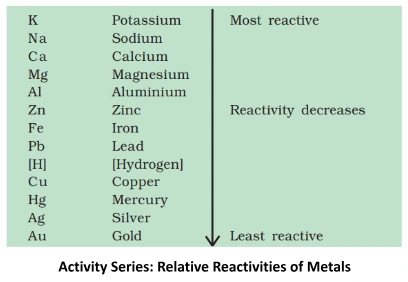
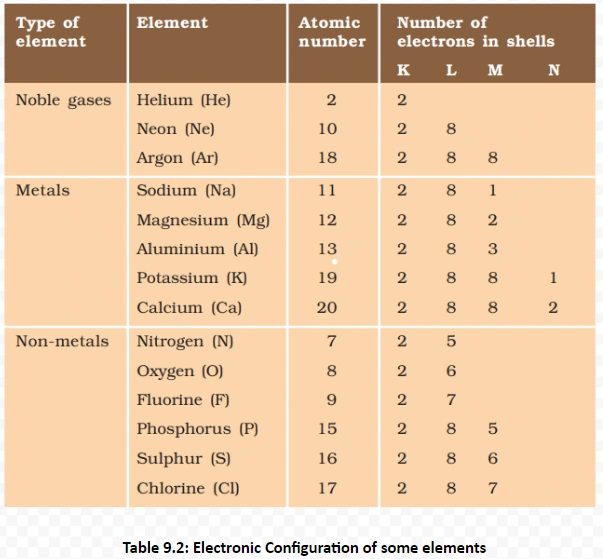
Understanding these properties Of metals and its Conductivity provides insights into the diverse behaviors of ionic compounds, influencing their applications across various scientific and industrial domains.
<div class="new-fform">
</div>