![]() 16 Dec 2023
16 Dec 2023
Extraction of metals is a fundamental process that unveils the treasures hidden within the Earth’s crust.
Corrosion:
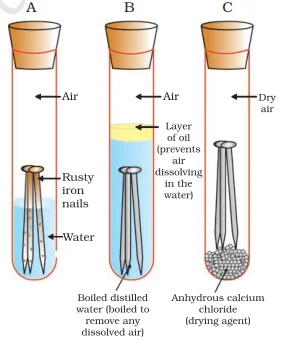
Rusting:
4Fe(s)+3O2 (from air)+xH2O(moisture)→2Fe2O3. xH2O(rust)
4Fe(s)+3O2 (from air)+xH2O(moisture)→2Fe2O3. xH2O(rust)
Oxidative Interactions of Copper with Atmospheric Gases: Formation of Green Copper Carbonate and Hydroxide
Cu(s)+H2O(moisture)+CO2(from air)→CuCO3.Cu(OH)2(green)
The Mechanism Behind Silver Corrosion in the Presence of Hydrogen Sulphide
Ag(s) + H2S(from air) →Ag2S(black) + H2(g)

Exploring the Unique Physical Properties of Nonmetals: An In-Depth Analysis
| Physical Properties of Nonmetals | Exceptions in Physical Properties |
|---|---|
|
|
<div class="new-fform">
</div>