Learn about Chlorofluorocarbons (CFCs), synthetic compounds once widely used in industrial applications. Discover how their stability and versatility led to their popularity, but also led to ozone layer depletion and environmental concerns. Understand the international efforts to phase out CFCs and the impact of their legacy on our environment.
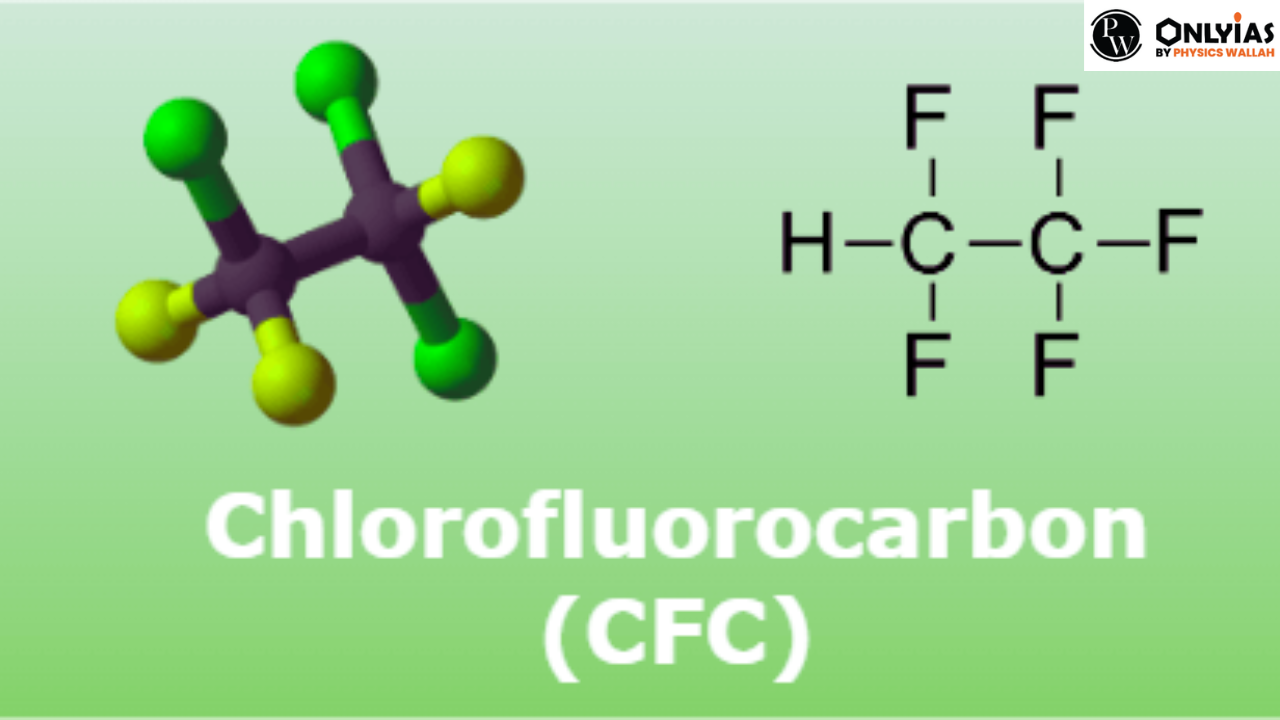
CFC stands for “Chlorofluorocarbon.” Chlorofluorocarbons are chemical compounds that were commonly used in various industries, including refrigeration, air conditioning, and aerosol propellants. However, their use has significantly declined due to their harmful impact on the ozone layer and the environment.
| CFC Full Form | |
|---|---|
| Full Form | Chlorofluorocarbon |
| Chemical Formula | Composed of carbon, chlorine, and fluorine atoms |
| Stability | Highly stable under normal conditions |
| Toxicity | Initially considered non-toxic |
| Flammability | Non-flammable |
| Reactivity | Low reactivity with other chemicals |
| Ozone Impact | Contribute to ozone depletion in the stratosphere |
Chlorofluorocarbons (CFCs) are a group of synthetic organic compounds that consist of carbon, chlorine, and fluorine atoms. They were initially developed in the early 20th century and gained widespread use in various industrial and commercial applications due to their unique properties. CFCs are known for being stable, non-toxic, non-flammable, and having low reactivity with other chemicals.
Chlorofluorocarbons (CFCs) have several distinct properties that made them attractive for various industrial applications. However, these properties also contributed to their negative environmental impact. Here are some key properties of CFCs:
The full form of “CFC” is “Chlorofluorocarbon.” CFCs are a group of synthetic organic compounds composed of carbon, chlorine, and fluorine atoms. They were commonly used as refrigerants, propellants, and solvents, but their use has been largely phased out due to their detrimental impact on the ozone layer and the environment.
Chlorofluorocarbons (CFCs) are a type of man-made chemical compound that gained widespread industrial use in the mid-20th century. Composed of carbon, chlorine, and fluorine atoms, CFCs were initially hailed for their stability, non-toxicity, and versatility in various applications. However, their harmful effects on the ozone layer and the environment later became a cause for significant concern.
CFCs were extensively utilized as refrigerants in air conditioning and refrigeration systems, as propellants in aerosol sprays, and as solvents in industries. Their low reactivity and stability allowed them to remain in the atmosphere for extended periods, eventually rising to the stratosphere. Here, they were exposed to ultraviolet (UV) radiation, which led to the release of chlorine atoms. These chlorine atoms played a destructive role in the ozone layer, a crucial part of the Earth’s atmosphere that shields us from harmful UV rays.
Ozone depletion caused by CFCs and other ozone-depleting substances prompted international action to address the issue. The Montreal Protocol, signed in 1987, aimed to phase out the production and consumption of CFCs and similar substances. This collective effort has led to a gradual reduction in CFC emissions and a recovery of the ozone layer in certain regions.
Scientists’ understanding of the adverse impact of CFCs on the environment also served as a catalyst for the development and adoption of more environmentally friendly alternatives. These alternatives, such as hydrochlorofluorocarbons (HCFCs) and hydrofluorocarbons (HFCs), have lower ozone depletion potential and less harmful effects on the environment.
The story of CFCs serves as a poignant reminder of the delicate balance of Earth’s ecosystems and the need for responsible technological innovation. The global response to the CFC issue demonstrates the capacity for international cooperation in addressing environmental challenges and safeguarding the planet for future generations.
Chlorofluorocarbons (CFCs) were once widely used in various industrial and commercial applications due to their unique properties, such as stability, low reactivity, and non-toxicity. However, their use has significantly declined and, in many cases, has been phased out due to their harmful impact on the ozone layer and the environment. Here are some historical applications of CFCs:
Chlorofluorocarbons (CFCs) have significant negative impacts on the environment, primarily due to their role in ozone depletion and their contribution to global warming. Here are the main ways in which CFCs affect the environment:
Due to the severe environmental consequences of CFCs, international efforts were made to reduce their production and use. The Montreal Protocol, signed in 1987, aimed to phase out the production and consumption of ozone-depleting substances, including CFCs. This agreement has been successful in reducing CFC emissions and allowing the ozone layer to slowly recover. However, the impacts of historical CFC emissions continue to affect the environment, underscoring the importance of continued vigilance and responsible management of ozone-depleting substances.
| Related Links | |
|---|---|
| RBI Full Form | PCS Full Form |
| GATT Full Form | TRAI Full Form |
Chlorofluorocarbons (CFCs) are synthetic organic compounds composed of carbon, chlorine, and fluorine atoms. They were once widely used in various industrial applications due to their stability and versatility.
CFCs were used as refrigerants in air conditioning and refrigeration systems, propellants in aerosol sprays, blowing agents for foams, and solvents in various industrial processes.
Ozone depletion refers to the thinning of the ozone layer in the stratosphere due to the breakdown of ozone molecules by chlorine and bromine atoms. This depletion allows more harmful UV radiation to reach the Earth's surface.
CFCs contribute to ozone layer depletion, which increases UV radiation exposure and poses risks to human health, ecosystems, and the environment. They also have a high global warming potential, contributing to climate change.
Safer alternatives, such as hydrochlorofluorocarbons (HCFCs) and hydrofluorocarbons (HFCs), have been developed to replace CFCs in various applications. These alternatives have lower or no ozone-depleting potential.

<div class="new-fform">
</div>