Context:
Recently, the Centre for Science and Environment (CSE) organized a webinar titled ‘The Crisis of Antibiotic Research and Development’ to discuss the issue of Antimicrobial Resistance (AMR).
More on the News:
- Increasing Resistance: Webinar highlights how the burden of antibiotic resistance is increasing and existing antibiotics are becoming ineffective.
- Ineffective: Most antibiotics developed over the last decade are not novel enough and insufficient to treat multidrug resistant bacteria
- Lack of R&D: The global antibiotic pipeline for new antibiotics is weak, particularly against Gram-negative priority pathogens.
- Small-and-medium scale antibiotic developers are trying to fill this void but are facing challenges and need support
- Reforms Needed: To stimulate the antibiotic R&D ecosystem for sustainable and equitable antibiotic access.
- These reforms include greater public financing, coordinated response from national governments, balanced public-private partnerships in antibiotic R&D.
- Global Public Good: Antibiotics can be considered a ‘global public good’.
About Antimicrobial Resistance (AMR)
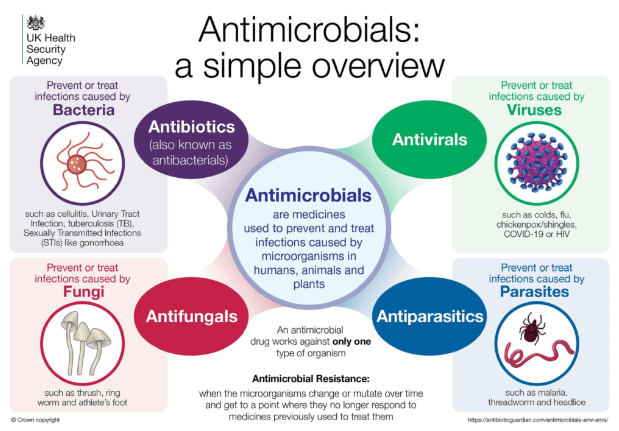
- It occurs when bacteria, viruses, fungi and parasites change over time and no longer respond to medicines making infections harder to treat and increasing the risk of disease spread, severe illness and death.
- As a result of drug resistance, antibiotics and other antimicrobial medicines become ineffective and infections become increasingly difficult or impossible to treat.
Global Severity of AMR:
- WHO has declared AMR as one of the top global public health threats.
- The United Nations has also called it a global health emergency.
- Mortality: In 2019, antibiotic-resistant infections caused 1.27 million deaths worldwide, with an overall 4.95 million deaths from associated complications.
- Financial Burden: AMR may cause a global annual GDP loss of $3.4 trillion by 2030 and it may push 24 million people into extreme poverty.
Severity of AMR in India
- AMR (Antimicrobial resistance) capital of the world: India has the highest infectious disease burden in the world, including infections due to multi-resistant pathogens.
- It is also the world’s largest consumer of antibiotics in terms of total volume.
- In 2019, 2,807 million packs of anti-infectives were sold in India, of which systemic antibiotics comprised 2,165 million packs (77.1 per cent).
- AMR adds to the burden of communicable diseases and strains the health systems of a country.
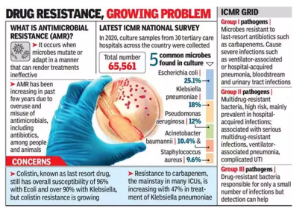 An Indian Council of Medical Research (ICMR) study in 2022 showed that the resistance level increases from 5% to 10% every year for broad-spectrum antimicrobials.
An Indian Council of Medical Research (ICMR) study in 2022 showed that the resistance level increases from 5% to 10% every year for broad-spectrum antimicrobials. - An Indian Network for Surveillance of Antimicrobial Resistance (INSAR) study indicated a high rate of resistance to commonly used drugs such as ciprofloxacin, gentamicin, co-trimoxazole, erythromycin and clindamycin.
Causes of Antimicrobial Resistance (AMR) in India:
- Overuse and misuse of antibiotics: According to Lancet Infectious Diseases journal, India is the world’s largest consumer of antibiotics, with a 62% increase in consumption between 2000 and 2015.
- This overuse and misuse of antibiotics has contributed significantly to the development of AMR in India.
- Inadequate sanitation and hygiene: Poor sanitation and hygiene practices can lead to the spread of infections that require antibiotics.
- According to the latest WHO-UNICEF data, at least one-sixth of India’s rural population still defecate in the open and a quarter doesn’t have even basic sanitation access, which can lead to the spread of diseases like cholera, typhoid, and diarrhea.
- Over the Counter Sale of Drugs:Antibiotics are easily available in India without a prescription, and there are no regulations on their sale.
- This has led to their inappropriate use, with people using them for viral infections like colds and flu, which do not require antibiotics.
- Emergence of new strains of bacteria: The emergence of new strains of bacteria that are resistant to multiple antibiotics is a growing concern in India.
- A total of 378 new microbial species were discovered in India between 2008 and 2019, in places ranging from pristine glaciers to grimy mobile phone screens.
- Poultry and veterinary use of antibiotics: Antibiotics are commonly used in the poultry and veterinary industry in India to prevent and treat infections.
- However, this has led to the development of AMR in animals, which can then be transmitted to humans through food or direct contact.
- Antibiotic use in agriculture: The antibiotic colistin is widely used as a growth promoter in livestock.
- At least one in ten top producers of colistin for agricultural use is in India.
- This has led to the development of AMR in bacteria found in soil and water, which can then be transmitted to humans through food or water.
- Wastewater contamination: Wastewater from hospitals, pharmaceutical companies, and other sources can contain high levels of antibiotics and resistant bacteria.
- According to Lancet, antibiotic residues in wastewater and wastewater treatment plants (WTP) serve as “potential hotspots for the development of AMR.
- Antibiotic residues from water are likely to sweep into groundwater. It can pollute the environment during the production, consumption and disposal of drugs.
- Some studies have reported bacteria with high levels of resistance to broad-spectrum antibiotics in specific river locations and potable water sources.
- Lack of effective regulations: India does not have an effective drug quality surveillance system.
- Existing regulations are obsolete and many times allow different standards and quality systems for drugs that get exported, versus drugs consumed domestically.
Steps Taken by India to Prevent AMR:
- India’s National Action Plan on AMR (NAP-AMR) for 2017-2021: It addresses six critical issues:
- Creating awareness and understanding of AMR through effective communication, education and training.
- Strengthening knowledge and evidence through surveillance.
- Reducing the incidence of infection through effective infection prevention and control.
- Optimising the use of antimicrobial agents in health, animals and food.
- Promoting investments for AMR activities, research and innovations.
- Strengthening India’s leadership on AMR.
- In the line with NAP-AMR three states have launched their state action plan:
- Kerala has launched KARSAP.
- Madhya Pradesh has launched MP-SAPCAR.
- Delhi has launched SAPCARD.
- Delhi Declaration on the AMR: An inter-ministerial consensus was signed at the launch of NAP-AMR, by the ministers of the ministries concerned pledging their whole-hearted support in AMR containment.
- AMRSN: ICMR has established AMR surveillance and research network (AMRSN) comprising 30 tertiary care hospitals, both private and government, to generate evidence and capture trends and patterns of drug resistant infections in the country.
Way Forward
- One Health Approach: AMR requires a united multisectoral approach, which can be achieved through the One Health approach.
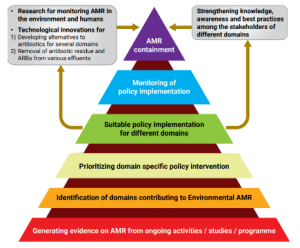 It brings together multiple sectors and stakeholders to work together in designing and implementing programmes, policies, legislation and research to attain better public health outcomes.
It brings together multiple sectors and stakeholders to work together in designing and implementing programmes, policies, legislation and research to attain better public health outcomes.
- 3C approach to R&D: Coordination, collaboration and communication are important because they help to ensure that efforts are not duplicated, resources are used efficiently, and stakeholders are aware of progress and challenges in the development of new antibiotics.
- Improve WASH (water, sanitation, hygiene) services: In large cities, waste management and proper WASH can be difficult, and metropolitan areas have been linked to numerous outbreaks of infectious diseases, notably those transmitted through the fecal-oral route.
- Other Steps to be taken:
| Short-Term Action Points |
Long-Term Action Points |
- Institutional and regulatory strengthening for improved effluent treatment and handling in sectors.
- Inclusion of residual antibiotic management in environmental policies and guidelines from the perspective of both manufacturing and use of antibiotics.
- Provision of subsidy or incentives to farmers for encouraging them to adopt alternatives to antibiotic growth promoters.
- Enhanced participation of pharmaceutical companies towards sustainable antibiotic manufacturing practices.
|
- Rational use of antibiotics in both humans and animals
- Multisectoral engagement for tracking and collection of AMR data from human health, animal health and the environment, applying the ‘One health’ approach
- Development of strong surveillance to monitor antibiotic residues and transmission of AMR bacteria from healthcare facilities, pharmaceutical effluents, food animals, water etc.
- Promotion of more research and technological innovation to optimize the use of antibiotics in certain sectors as well as to reduce the incidence of AMR
- Making the engagement of microbiologists and environmental specialists in the sectors contributing to environmental AMR mandatory, for time-to-time monitoring and reporting of the resistance pattern of the different bacteria in the environmental samples
|
Global Efforts in Prevention of AMR
- WHO Global Action Plan on Antimicrobial Resistance
- It is a comprehensive strategy that aims to tackle the growing problem of antimicrobial resistance, which is a major threat to global health.
- Objectives:
- To improve awareness and understanding of antimicrobial resistance through effective communication, education, and training.
- To strengthen the knowledge and evidence base through surveillance and research.
- To reduce the incidence of infection through effective sanitation, hygiene, and infection prevention measures.
- To optimize the use of antimicrobial medicines in human and animal health.
- To develop the economic case for sustainable investment that takes account of the needs of all countries, and increase investment in new medicines, diagnostic tools, vaccines, and other interventions.
- To improve global governance of antimicrobial resistance.
- Global Antimicrobial Resistance and Use Surveillance System (GLASS)
- It is a global system for the standardised collection, analysis and sharing of data on antimicrobial resistance (AMR) and antimicrobial use (AMU) at a national, regional and global level.
- Global Antibiotic Research and Development Partnership (GARDP)
- GARDP is a not-for-profit global partnership developing treatments for drug-resistant infections that pose the greatest threat to health. GARDP works across sectors to ensure equitable access to treatments and promote their responsible use.
- One Health Quadripartite: In 2021, the Food and Agriculture Organization, UN Environment Programme (UNEP), WHO and World Organisation for Animal Health joined forces to constitute the One Health Quadripartite to combat health risks, including AMR, at the human, animal and plant and ecosystems interface.
- WHO Essential Medicines List categorizing Antimicrobial in three groups:
- ACCESS – first and second choice antibiotics for the empiric treatment of most common infectious syndromes;
- WATCH – antibiotics with higher resistance potential whose use as first and second choice treatment should be limited to a small number of syndromes or patient groups; and
- RESERVE – antibiotics to be used mainly as ‘last resort’ treatment options.
- Muscat Ministerial Manifesto on AMR:
- It was signed by health ministers from around the world in 2017, committing to take action to address the growing threat of AMR. It outlines three global targets:
-
- Reducing the total amount of antimicrobials used in agrifood systems by at least 30 per cent-50 per cent by 2030.
- Preserving critically important antimicrobials for human medicine and ending the use of medically important antimicrobials for growth promotion in animals.
- Ensuring that ‘Access’ group antibiotics (a category of antibiotics that are affordable, safe and have a low AMR risk) represent at least 60 per cent of overall antibiotic consumption in humans by 2030.
|
News Source: DTE
![]() 10 Aug 2023
10 Aug 2023

 An Indian Council of Medical Research (ICMR) study in 2022 showed that the resistance level increases from 5% to 10% every year for broad-spectrum antimicrobials.
An Indian Council of Medical Research (ICMR) study in 2022 showed that the resistance level increases from 5% to 10% every year for broad-spectrum antimicrobials.  It brings together multiple sectors and stakeholders to work together in designing and implementing programmes, policies, legislation and research to attain better public health outcomes.
It brings together multiple sectors and stakeholders to work together in designing and implementing programmes, policies, legislation and research to attain better public health outcomes.