![]() 22 Jan 2024
22 Jan 2024
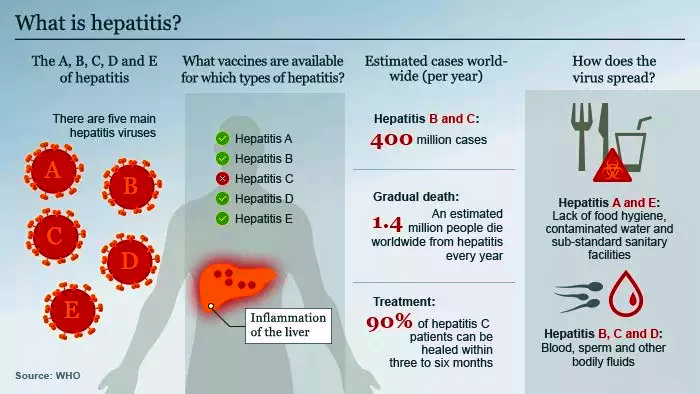
Recently, Hyderabad-based Indian Immunologicals Ltd (IIL) has launched India’s first indigenously developed Hepatitis A vaccine Havisure®
Also Read:
News Source: Deccan Herald
| Must Read | |
| NCERT Notes For UPSC | UPSC Daily Current Affairs |
| UPSC Blogs | UPSC Daily Editorials |
| Daily Current Affairs Quiz | Daily Main Answer Writing |
<div class="new-fform">
</div>