This article sheds light on the biobank and its evolution for Precision medicine.
- Precision medicine is revolutionising personalised healthcare, relying on advanced technologies like genomics, gene-editing, and mRNA therapeutics.
Evolution and Status of Precision Medicine in India
- Introduction: Precision medicine focuses on personalised healthcare, and it has rapidly grown since the Human Genome Project.
- Growth of Precision Medicine in India
- The precision medicine market in India is expected to grow at a rate of 16% CAGR and surpass $5 billion by 2030.
- It contributes to 36% of India’s bioeconomy, with advancements in cancer treatment, gene editing, and biologics.
- Key Developments in Precision Medicine
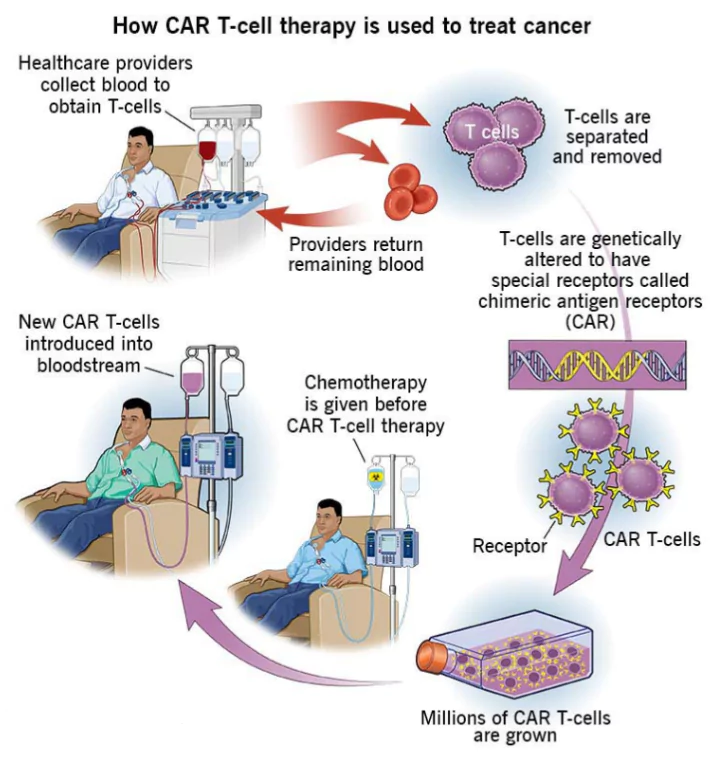
- NexCAR19: India’s first domestically developed CAR-T cell therapy was approved in 2023, marking a significant milestone.
- CAR-T cell therapy is used to treat cancer.
- AI Collaborations: Partnerships, such as those between Siemens Healthineers and the Indian Institute of Science, focus on using artificial intelligence for cancer treatments and advancing precision medicine.
Enroll now for UPSC Online Course
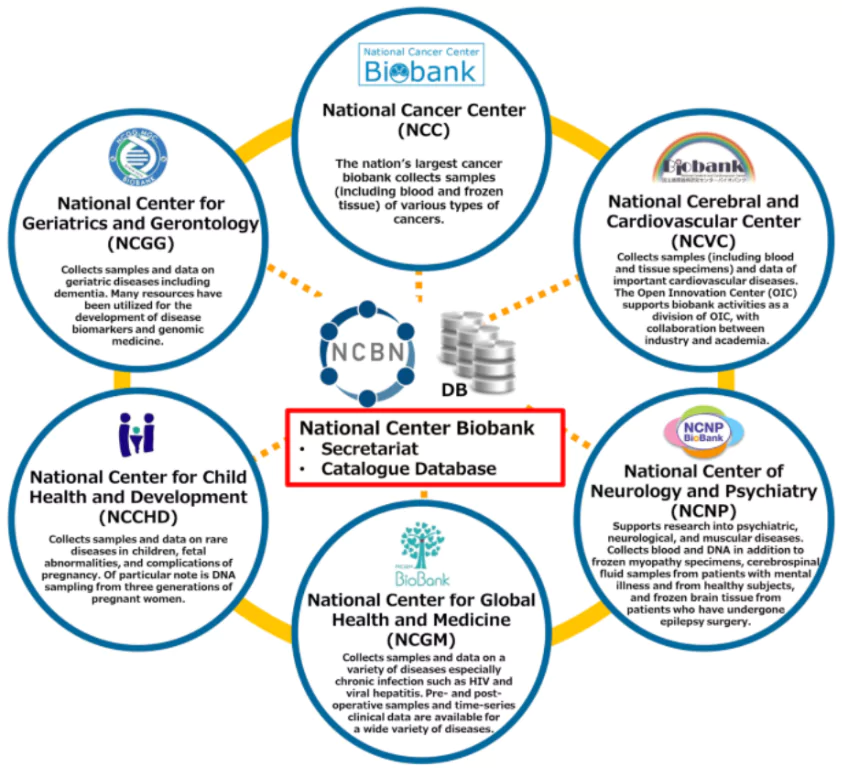
About Biobanks
A biobank is a repository that stores biological samples, such as blood, DNA, cells, tissues, and organs, for research purposes.
- They play a vital role in precision medicine by providing valuable information for studying diseases and developing targeted treatments.
Importance of Biobanks
- Large-Scale Research: Biobanks provide researchers with access to a vast collection of samples, enabling them to conduct large-scale studies that would be difficult or impossible with smaller sample sizes.
- Genomics and Personalized Medicine: Biobanks are essential for genomics research, which aims to understand the genetic basis of diseases.
- This knowledge can lead to the development of personalised treatments tailored to individual patients’ genetic makeup.
- Disease Biomarkers: By analysing samples from large groups of people, researchers can identify genetic variations associated with specific diseases.
- These variations can serve as biomarkers for early diagnosis and targeted treatment.
- Genome India:
- It is a national project, funded by the Department of Biotechnology.
- Objective: It aims to sequence 10,000 genomes from healthy individuals across India.
- Phenome India:
- It is the initiative of the Council of Scientific and Industrial Research (CSIR).
- Launch date: 2023 (Dec)
- Objective: This project aims to develop India-specific risk prediction models for diseases like liver diseases, and cardiac conditions.
- The Paediatric Rare Genetic Disorders (PRaGeD) mission.
- It is a pan India initiative.
- Funded by: Department of Biotechnology (DBT), Ministry of Science and technology.
- Objective: Developing art facility to study Paediatric Rare Genetic Diseases.
|
Biobank Regulations in India
India currently has 19 biobanks, and initiatives like Genome India and Phenome India aim to improve disease prediction and treatment.
- Regulatory challenges
- However, India lacks comprehensive biobank regulations, creating issues related to:
- Informed consent: Participants are unsure how their data will be used.
- Privacy risks: Genetic data could lead to discrimination.
- Regulatory gaps: There is no single authority to regulate biobanks, and misconduct may occur.
- Ethical concerns: There is a need to resolve issues such as exploitation, stigma, and discrimination based on genetic information.
- Sample quality and integrity: Maintenance of quality and integrity of biological samples is necessary over time.
- It requires necessary protocols and optimal storage conditions for preventing it from degradation and contamination.
- Legal Framework Governing Biobanks in India
- Lack of Comprehensive Legislation
- India does not have specific laws for biobanks.
- Existing guidelines are not enforceable, leading to regulatory gaps.
- National Ethical Guidelines by ICMR
- The Indian Council for Medical Research (ICMR) has issued ethical guidelines for biomedical research involving humans.
- These guidelines are not legally binding and do not fully address long-term storage or data sharing for biobanks.
- Department of Biotechnology (DBT) Standards
- The DBT has set practices for data storage and analysis.
- However, these are not enforceable and do not adequately cover issues like informed consent and privacy.
- Absence of a Single Regulatory Authority
- India lacks a dedicated regulatory authority for biobanks.
- This results in inconsistencies and limited oversight in biobanking activities.
Check Out UPSC CSE Books From PW Store
Global Standards and India’s Path Forward
- Countries like the U.S., U.K., and Japan have stringent biobank laws covering data protection, consent, and privacy.
- India needs similar laws to protect participant rights and ensure ethical research practices.
|
![]() 15 Oct 2024
15 Oct 2024

