Scientists from the Institute of Nano Science and Technology (INST), part of the Department of Science and Technology (DST), developed a new copper-based catalyst.
- The catalyst has a unique star-like nanostructure and is designed to make industrial chemical reactions more sustainable.
About Copper-based Catalyst
- This catalyst is created by growing copper oxide nanostructures on a sporopollenin template.
- It offers a sustainable pathway for industrial chemical reactions.
- These catalysts can be considered green catalysts due to Abundant, Low Toxicity, Efficiency, and its Reusability.
Enroll now for UPSC Online Classes
What is Sporopollenin?
- It is a biological polymer.
- Found in: outer walls of pollen grains and spores.
- It has a bowl-like outer structure.
- This structure provides a framework for the controlled growth of copper oxide nanostructures.
|
- It is efficient in water without additives and can be reused multiple times
- Applications: Useful in organic reactions, environmental remediation, nanoscale electronics, and surface-enhanced Raman spectroscopy (SERS).
- Advantages of Copper-Based Catalysts
- Copper-based catalysts are widely used due to their unique properties and benefits:
- Abundance and Cost-Effectiveness
- Easily Available: Copper is naturally abundant and readily accessible.
- Low Cost: It is an inexpensive metal, making it a cost-effective choice for catalytic applications.
Green catalysts
- Green catalysts are substances that accelerate chemical reactions while minimizing the environmental impact.
- They are designed to promote sustainability and reduce the use of hazardous materials in industrial processes.
|
-
-
- Multiple Oxidation States
- Flexible in Reactions: Copper exists in various oxidation states (Cu⁰, Cu⁺, Cu²⁺, Cu³⁺), enabling it to participate in a variety of chemical reactions.
- Versatile Applications
- Redox Reactions: Widely used in oxidation-reduction processes.
- CO Oxidation: Effective in the conversion of carbon monoxide to carbon dioxide.
- Selective Oxidation: Facilitates the selective oxidation of organic compounds.
- Electrochemical Reactions: Plays a key role in hydrogen evolution reactions (HER).
Check Out UPSC NCERT Textbooks From PW Store
About Nano Catalyst
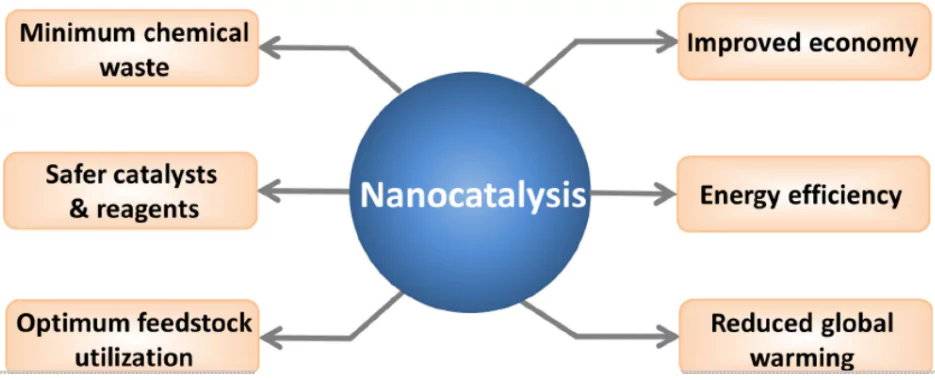
- A nano catalyst is a material made up of nanoparticles that speeds up chemical reactions without being consumed.
- Size: Operates at the nanoscale, with particle sizes ranging from 1 to 100 nanometers.
- Key Features of Nano Catalysts
- High Surface Area: Nanoparticles provide a large surface area, increasing the number of active sites for chemical reactions.
- Selectivity: Nano catalysts can be tailored to target specific reactions, reducing unwanted side reactions.
- Environmentally Friendly: Many are designed to follow green chemistry principles, requiring less energy and avoiding harsh chemicals.
- Applications of Nano Catalysts
- Industrial Chemical Reactions : Improve process efficiency while minimizing environmental impact.
- Environmental Remediation: Help in removing pollutants from air and water.
- Pharmaceutical Industry: Enable cleaner and more sustainable drug synthesis.
- Energy Production: Support the generation of clean energy sources like hydrogen.
- Limitations of Nano Catalysts
- High reaction time: Nanocatalysts can have longer reaction time.
- It is very expensive.
- Toxicity risks: Its use and disposal is risky to human health and environment.
- High sensitivity: It can lose its effectiveness under some conditions such as extreme temperatures and PH levels.
![]() 11 Jan 2025
11 Jan 2025


