The Indian Council of Medical Research (ICMR) has invited Expressions of Interest (EoI) from Indian organisations, companies, and manufacturers for the development and production of monoclonal antibodies (mAbs) against the Nipah virus (NiV).
What is Monoclonal Antibodies?
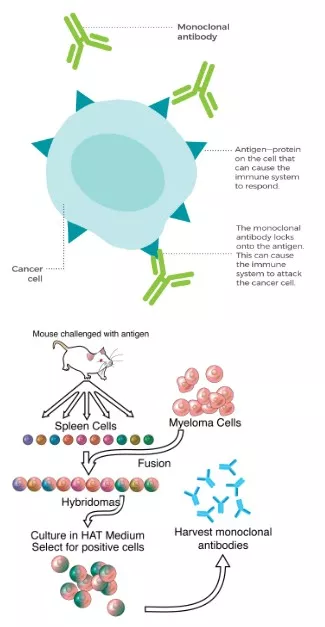
- Monoclonal antibodies are laboratory-produced molecules designed to mimic the immune system’s ability to fight harmful pathogens such as viruses and bacteria.
- They are called “monoclonal” because they are made by identical immune cells (clones) derived from a single parent cell — hence, they recognize and bind to one specific target (antigen).
- Working Mechanism:
- They bind to a specific antigen (target molecule) on the pathogen or cell.
- This binding can neutralize toxins, block receptors, or mark cells for destruction by other immune components.
- Production:
- They are created using the hybridoma technique, where a specific B-cell (B-cells, also known as B lymphocytes, are a type of white blood cell) producing the desired antibody is fused with a myeloma (cancer) cell.
- This creates a hybridoma that can divide indefinitely and produce large quantities of the same antibody.
- Application:
- Medical treatment: Used in cancer therapy (e.g., Rituximab, Trastuzumab), autoimmune diseases (e.g., Adalimumab), and viral infections (e.g., COVID-19 monoclonal antibodies).
- Diagnosis: Used in rapid antigen tests and laboratory diagnostics.
- Research: To identify or isolate specific molecules in cells and tissues.
- Advantages:
- Highly specific and targeted action.
- Can reduce side effects compared to broad-spectrum drugs.
- Useful when vaccines or antivirals are unavailable or ineffective.
- Limitations:
- Expensive to produce and store (require cold chain).
- May cause immune reactions in some cases.
- Limited effectiveness if the target antigen mutates (as in some viral strains).
- Application in Nipah Control:
- Post-exposure prophylaxis: For healthcare workers, family members, and laboratory personnel exposed without adequate protection.
- Therapeutic use: Administered early, mAbs can reduce viral load, limit disease progression, and improve survival outcomes.
About the ICMR Initiative
- Objective: Develop, produce, and stockpile indigenous monoclonal antibodies against Nipah virus for therapeutic and prophylactic use during outbreaks.
- Implementing Agency: ICMR and ICMR–National Institute of Virology (NIV), Pune, will provide technical guidance, research support, and oversight at all phases.
- Collaboration Model: ICMR will partner with Indian industry and biotech firms to create a domestic monoclonal antibody platform for scalable production.
- Current Status:
- Animal trials successfully completed using indigenously developed mAbs.
- ICMR–National Institute of Virology (NIV), Pune has initiated advanced R&D in this domain.
- Rationale:
- High Fatality Rate: Ranges between 40% and 75%, depending on clinical management and outbreak response.
- Recurring Outbreaks in India: Repeated since 2001, with frequent spillovers in Kerala (notably in 2018, 2021, 2023, 2024, and 2025).
- Current Gaps:
- No approved vaccine or antiviral therapy.
- Reliance on imported or experimental antibody stocks during past outbreaks caused deployment delays.
- Strategic Need: Building domestic mAb capacity ensures self-reliance and rapid deployment during emergencies.
About Nipah Virus (NiV)
- Nature:
- Nipah Virus (NiV) is a zoonotic virus (transmitted from animals to humans) that can also spread through contaminated food or from person to person.
- It causes severe respiratory illness and encephalitis (brain inflammation) in humans.
- Classified as a highly pathogenic virus due to its high fatality rate and epidemic potential.
- Origin & History:
- First identified: In Malaysia, 1998–99 among pig farmers.
- Name: Derived from Sungai Nipah, a Malaysian village where it was first detected.
- Reservoir host: Fruit bats of the genus Pteropus (also called flying foxes).
- Transmission:
- Animal to Human: Contact with infected bats or pigs; Consumption of raw date palm sap contaminated by bat saliva or urine.
- Human to Human: Through respiratory droplets, body fluids, or contaminated surfaces, especially in healthcare settings.
- Fatality rate: 40–75%, depending on outbreak response and healthcare access.
- Strain in India: The Bangladesh clade (NiV-B) — known for frequent person-to-person transmission and higher mortality than the Malaysian strain.
![]() 4 Nov 2025
4 Nov 2025


