![]() 12 Feb 2026
12 Feb 2026
English
हिन्दी

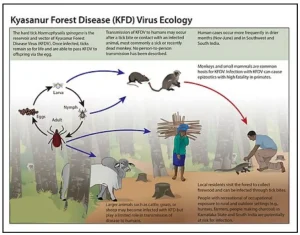
Indian Council of Medical Research (ICMR) has initiated Phase I clinical trials of an improved indigenous vaccine against Kyasanur Forest Disease (KFD) following CDSCO approval.
Check Out UPSC CSE Books
Visit PW Store
About Central Drugs Standard Control Organization (CDSCO)
|
|---|
<div class="new-fform">
</div>