![]() 16 Dec 2023
16 Dec 2023
Food, clothes, medicines, books, or many other things are all based on this versatile element carbon. In addition, all living structures are carbon based. Carbon is available in nature, The importance of carbon and its compounds in nature is immense.
About Carbon and its compounds
Carbon’s Tetravalency and Covalent Bonds: A Comparative Analysis
Bonding in Hydrogen:
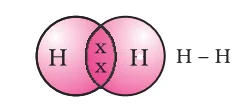
Hydrogen Bonding: Forming Covalent Bonds in Hydrogen Molecules
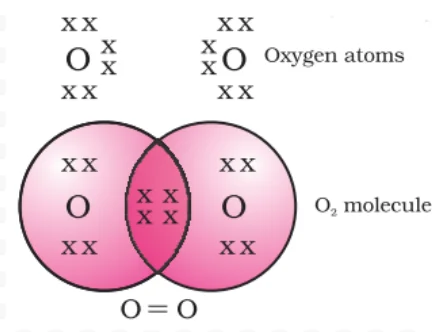
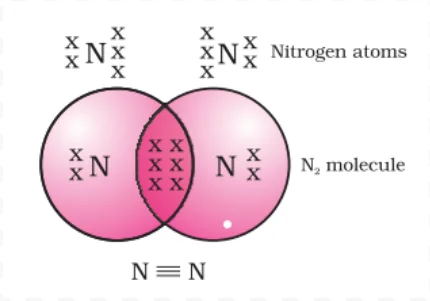
Triple Bonding in Nitrogen: Achieving Noble Gas Configuration through Shared Electrons
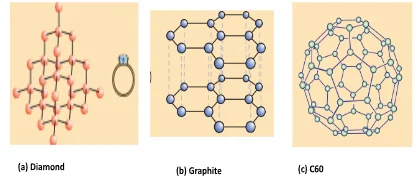

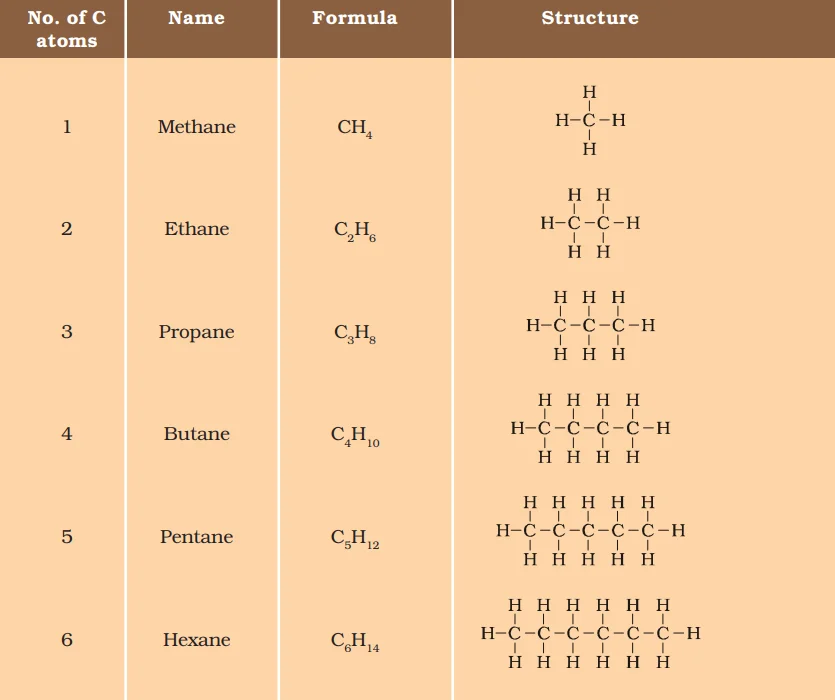
Functional Diversity in Carbon and its Compounds: Exploring Hydroxyl, Aldehyde, Ketone, Carboxyl, and Halogen Groups
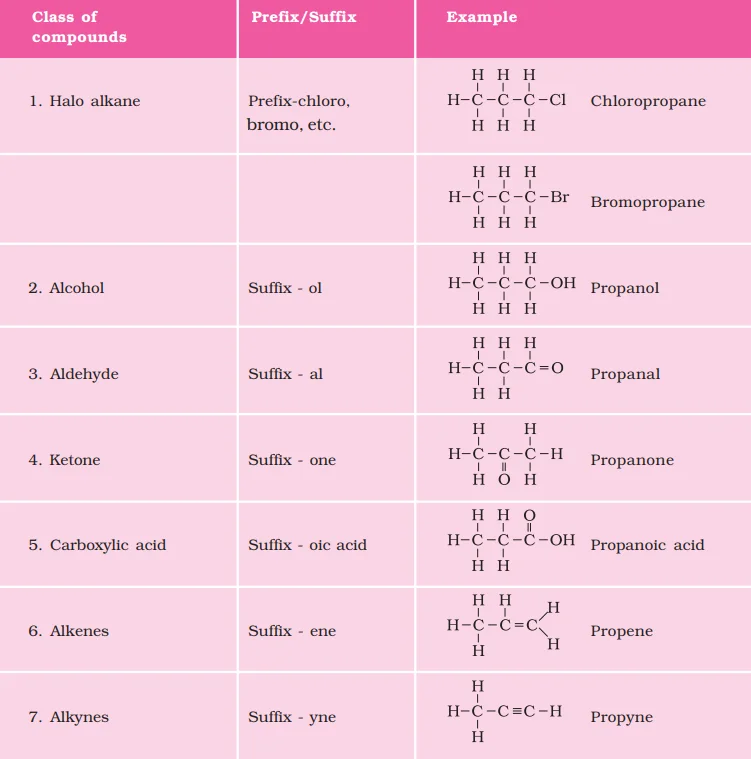
<div class="new-fform">
</div>