![]() 18 Dec 2023
18 Dec 2023
Formation of coal and petroleum

Why do substances burn with or without a flame?
This is because a flame is only produced when gaseous substances burn. When wood or charcoal is ignited, the volatile substances present evaporate and burn with a flame in the beginning.
A luminous flame is seen when the atoms of the gaseous substance are heated and start to glow. The color produced by each element is a characteristic property of that element.
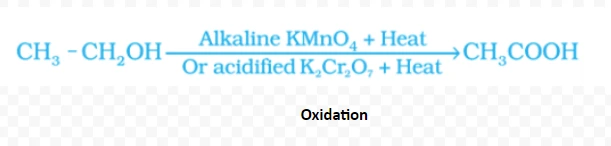
2C2H5OH + 2Na → 2C2H5ONa + H2(↑)
CH3CH2OH → CH2=CH2 + H2O
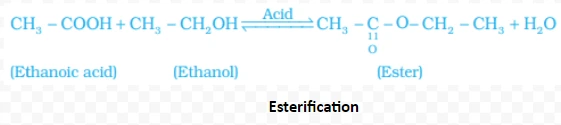
NaOH + CH3COOH → CH3COONa + H2O
2CH3COOH + Na2CO3 → 2CH3COONa + H2O + CO2
CH3COOH + NaHCO3 → CH3COONa + H2O + CO2
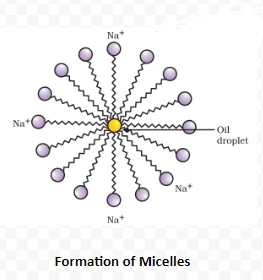
How do alcohols affect living beings?
Alcohol as a fuel
<div class="new-fform">
</div>