![]() 18 Dec 2023
18 Dec 2023
Physical and chemical changes are fundamental concepts in chemistry. Physical changes involve alterations in the state or form of matter without changing its chemical composition. In contrast, chemical changes result in a new substance with distinct properties. Chemical reactions, such as combustion or rusting, exemplify this transformation.
Metalloids: These are elements showing properties between those of metals and nonmetals, examples are boron, silicon, germanium.
|
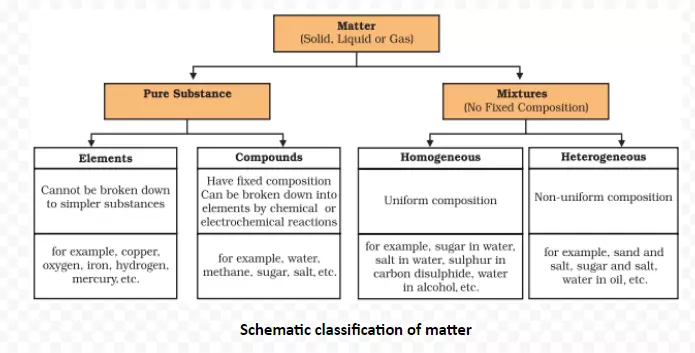
Mixtures and Compounds |
|
| Mixtures | Compounds |
| Elements or compounds just mix together to form a mixture and no new compound is formed. | Elements react to form new compounds. |
| A mixture has a variable composition. | The composition of each new substance is always fixed. |
| A mixture shows the properties of the constituent substances. | The new substance has totally different properties. |
| The constituents can be separated fairly easily by physical methods. | The constituents can be separated only by chemical or electrochemical reactions. |
Are Compounds Formed through Elemental Transformation?
Meaning: A compound is a substance composed of two or more elements, chemically combined with one another in a fixed proportion.
<div class="new-fform">
</div>