The Food Safety and Standards Authority of India (FSSAI) banned the use of select antibiotics in the production of meat, poultry, eggs, milk, milk products, and aquaculture to address the growing threat of antimicrobial resistance (AMR).
Key Highlights of the Ban
- Objective: To prevent the transfer of antibiotic resistance genes (ARGs) from animals to humans through food.
Antibiotic Resistance Genes
- (ARGs) are genetic elements that enable bacteria to survive exposure to antibiotics by neutralizing or expelling the drugs.
- These genes can spread between bacteria through horizontal gene transfer, accelerating the development of antibiotic-resistant infections in humans and animals.
|
- Background: India reaffirmed its commitment to the Muscat Ministerial Manifesto on AMR (2022), aiming to reduce antimicrobial use in agrifood systems by 30–50% by 2030.
- Scope: The ban applies across all major livestock and aquaculture systems.
- Impact: Aims to preserve antibiotics critical for human health and phase out their use for animal growth promotion.
Use of Antibiotic as growth promoter:
- Antibiotics used as antimicrobial growth promoters (AMGPs) alter gut microbiota by suppressing harmful bacteria, enhancing nutrient absorption, and reducing inflammation.
- This improves feed efficiency and weight gain in livestock.
- Global Status of AMGP Use: Since the 1940s, AMGPs were widely adopted, with the USFDA approving them in 1951.
- However, countries like Sweden and Denmark banned them early due to resistance risks, Denmark banned avoparcin in 1995 after links to vancomycin-resistant enterococci (VRE) emerged.
- Use in India:India is the fourth-largest user of antibiotics in food-producing animals.
- Resistance surveillance (2019–22) revealed high antibiotic resistance in aquaculture and poultry, including 91.3% of Staphylococcus aureus isolates resistant to penicillin, highlighting widespread misuse and urgent need for regulation.
Anti-Microbial Resistance (AMR)
- AMR occurs when microbes (bacteria, fungi, viruses, parasites) evolve to resist the effects of antimicrobial medicines, rendering treatments ineffective.
- AMR is developed in bacteria through ARGs which are transferred from animals to Humans and vice versa.
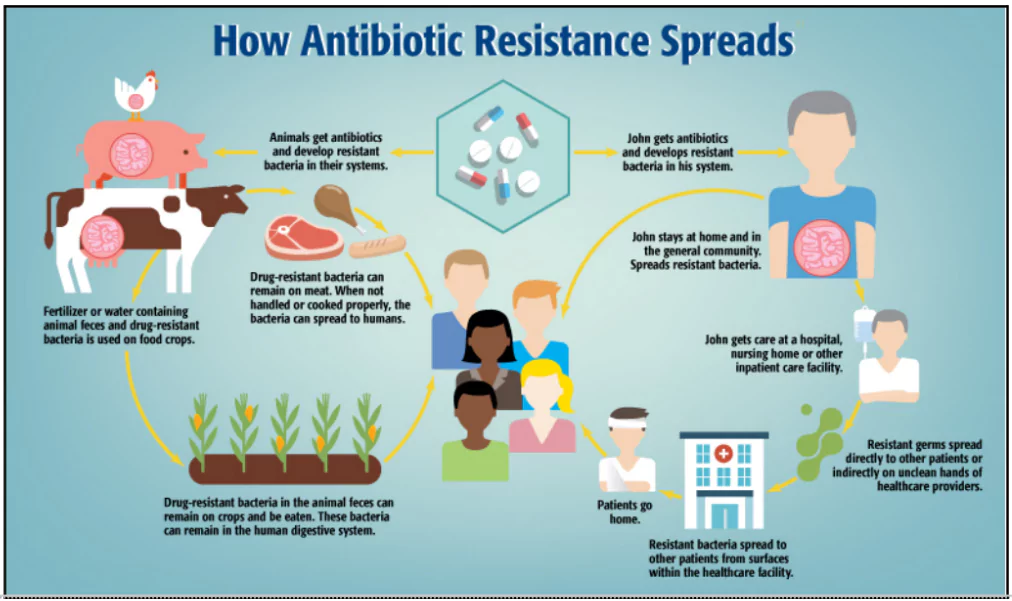 Examples of Resistant Pathogens:
Examples of Resistant Pathogens:-
- Methicillin-resistant Staphylococcus aureus (MRSA)
- Vancomycin-resistant enterococci (VRE)
- Drug-resistant Escherichia coli
- Multidrug-resistant tuberculosis (MDR-TB)
- Threat of AMR:
- Global Impact (WHO): Causes 700,000 deaths annually; projected to reach 10 million deaths per year by 2050 if unaddressed.
- India’s Burden: Among the top consumers of antibiotics in livestock.
- Studies show that more than 50% resistance to common antibiotics in fish, poultry, and pigs.
Some of the Banned Antibiotics
| Antibiotic |
Common Misuse |
Health Risk |
| Colistin |
Growth promoter in livestock |
Resistance development; undermines last-resort treatment options |
| Chloramphenicol |
Used in aquaculture and poultry |
Aplastic anemia in humans; banned in many countries |
| Sulphamethoxazole |
Infection prevention in livestock |
Allergic reactions; resistance in human pathogens |
| Carbadox |
Growth promoter in swine |
Carcinogenic; banned in multiple countries |
| Nitrofurans |
Used in aquaculture and poultry |
Carcinogenic; residues hazardous to consumers |
| Amoxicillin |
Broad-spectrum use in livestock |
Resistance in human medicine due to overuse |
| Cephalexin |
Treat infections in animals |
Encourages resistant bacterial strain development |
| Gentamicin |
Used in poultry and dairy |
Kidney toxicity from residues; resistance risk |
| Sulfamethazine |
Growth promoter and disease prevention |
Carcinogenic potential; unsafe residue levels |
| Sulfadimethoxine |
Used in aquaculture and poultry |
Hypersensitivity reactions; contributes to resistance |
Challenges in Tackling AMR
- Economic Dependence on Livestock Sector: India’s livestock sector saw a 7.9% CAGR between 2014–21, pushing antimicrobial use to boost productivity.
- Overuse of Antibiotics: Antibiotics like avoparcin were widely used for decades to increase feed efficiency but have led to resistant strains.
- Lack of Enforcement: Despite guidelines, over-the-counter antibiotic sales persist, often without prescription.
- Small-Scale Production: Backyard poultry and aquaculture setups complicate surveillance and regulation efforts.
- Insufficient Surveillance and Data: Limited infrastructure for real-time resistance tracking across all regions and sectors.
- Awareness Gaps: Misuse of antibiotics due to lack of awareness remains widespread among farmers and consumers.
Global Initiatives to Tackle AMR
- Global Action Plan on AMR (GAP-AMR) of WHO, 2015: Its goal is to ensure access to effective antimicrobials while reducing misuse.
- It Focuses on Surveillance, research, stewardship, and infection control.
- Global Antimicrobial Resistance and Use Surveillance System( GLASS), 2015: Developed by WHO to standardize global AMR surveillance.It assesses spread and impact of AMR to inform national policies.
- Muscat Ministerial Manifesto on AMR (2022): Commitment by countries including India to reduce antimicrobial use in food systems by up to 50% by 2030.
- It promotes preservation of essential human antibiotics.
- WHO AMR Research Agenda ( 2030): It Focuses on 40 research priorities in human and zoonotic AMR.
- It addresses both bacterial and fungal resistant pathogens.
National Initiatives to Tackle AMR
- National Programme on AMR Containment (India, 2013): The programme led by National Centre for Disease Control (NCDC) includes rational antimicrobial use, IPC practices, and awareness generation.
- National AMR Surveillance Network (NARS-Net): It is a network of 60 state labs across 33 States/UTs.
- It tracks resistance trends in 9 priority bacterial pathogens and Candida spp.
- Red Line Campaign: Public campaign discouraging over-the-counter use of red-line labelled antibiotics.
- It promotes prescription-based use only.
- Legal Framework in India:
- Drugs and Cosmetics Act, 1940 & Rules, 1945 regulate the manufacture, distribution, and sale of antibiotics, ensuring they are used only under medical supervision.
- Schedule H and H1: These schedules classify antibiotics that require a valid prescription for sale, limiting over-the-counter misuse.
- Central Drugs Standard Control Organization (CDSCO): CDSCO oversees the enforcement of drug regulations and ensures the quality and responsible distribution of antimicrobials across India.
Way Forward
- Strengthen Biosecurity and Animal Welfare Practices: Improve farm-level hygiene, isolate infected animals, and minimize disease introduction to reduce antibiotic dependence.
- Promote Use of Alternatives: Encourage safe substitutes like probiotics, prebiotics, bacteriophages, organic acids, and vaccines.
- Invest in Surveillance and Data Infrastructure: Enhance real-time AMR data sharing between states and improve lab capacities to support nationwide surveillance.
- Implement Stronger Regulatory Frameworks: Tighten controls on antibiotic sale and use in both human and veterinary medicine with strict penalties for violations.
- Expand Awareness and Community Engagement: Educate farmers, healthcare providers, and the public on AMR risks and safe practices through campaigns in local languages.
- Encourage Research and Innovation: Support R&D for new antimicrobials, diagnostic tools, and resistance-monitoring technologies.
![]() 29 Apr 2025
29 Apr 2025

 Examples of Resistant Pathogens:
Examples of Resistant Pathogens: