The ongoing controversy over Pertuzumab highlights that, despite legal protections, Evergreening still complicates the introduction of affordable biosimilars.
Roche Raises Concerns Over Zydus Trials of Breast Cancer Drug Pertuzumab
- Controversy of Pertuzumab: Pertuzumab is a popular breast cancer treatment.
- Roche, the world’s top seller of oncology drugs, has raised red flags against the clinical trials conducted by Indian drug maker Zydus Lifesciences on Perjeta (pertuzumab), its popular breast cancer treatment.
- The Swiss drugmaker, Roche, has alleged that 500 vials of the drug were sourced from questionable channels for clinical trials in India.
- Roche has reported concerns to India’s drug regulator, Central Drugs Standard Control Organisation (CDSCO), highlighting issues around proper sourcing and oversight in biosimilar trials.
- The Indian biopharmaceutical industry is one of the fastest growing globally and is valued at $60 billion.
- India is now ranked 39th in the Global Innovation Index, up from 81 in 2015.
Enroll now for UPSC Online Course
About Biopharmaceuticals
- Biopharmaceuticals are medicines derived or synthesised from living cells such as humans, animals, yeast, bacteria etc using biological processes, contrasting with conventional chemical-based drugs.
- They play a crucial role in treating chronic diseases like cancer and diabetes.
Components of Biopharmaceuticals
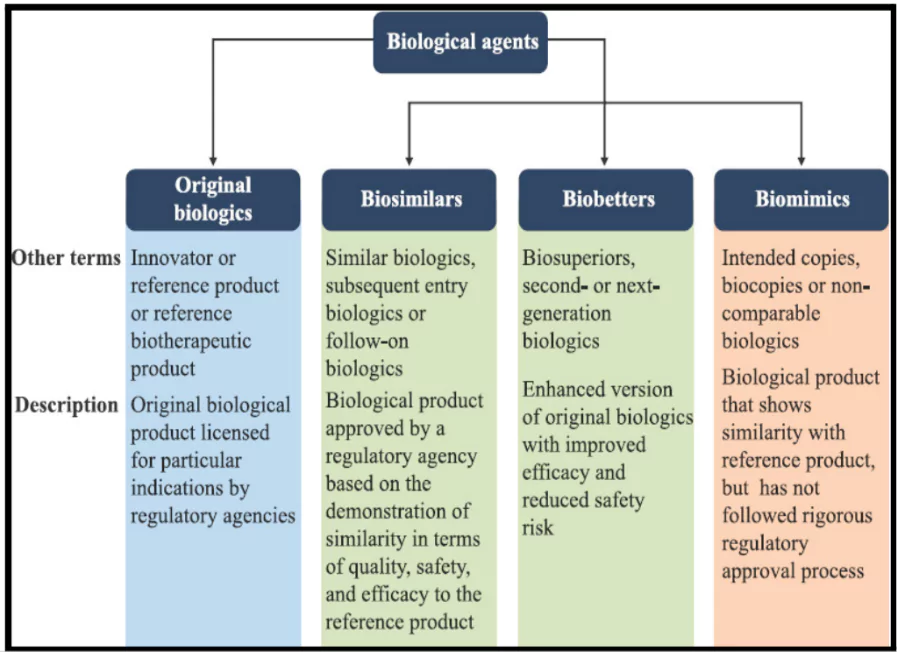
- Biologics: This term specifically refers to products derived from living cells or organisms, such as proteins, nucleic acids, or cells.
- They encompass a wide range of products, including: vaccines, blood components, gene therapy, tissues, proteins, like monoclonal antibodies and cell signalling proteins etc.
- Biosimilars: A Biosimilar is a biologic that is “similar” to another biologic medicine (known as a reference product) that has been cleared by the authorities for prescription by doctors.
- That is why they are also called ‘follow-on biologics’ and are used to treat the same disorders as the first biologic drug.
- Biosimilars closely match the reference product in safety, purity, and potency, though they may contain minor variations in inactive components. Example:
- Trastuzumab (Herceptin) – Used for treating breast cancer.
- Epoetin alfa (Epogen) – Used to treat anaemia, particularly in patients with chronic kidney disease.
- They offer new hope for patients by providing cost-effective alternatives to original biologics, which are often expensive and complex.
Difference between Biosimilars and Generics
| Aspect |
Biosimilars |
Generics |
| Nature of the Product |
- Highly similar to an approved biologic; made from living organisms; may have minor differences in inactive components.
|
- Exact copies of small-molecule drugs with the same chemical composition
|
| Regulatory Approval |
- Requires extensive studies to prove bio similarity and clinical performance.
|
- Approved based on bioequivalence to the branded drug.
- Does not require extensive clinical trials that were necessary for the original branded drug.
|
| Manufacturing Process |
- Complex production involves living cells; small changes can affect the final product.
|
- Simpler chemical synthesis; can be produced consistently.
|
| Market Dynamics |
- Newer market with increased competition and reduced prices, but higher development costs.
|
- Established market with significant price reductions upon entry.
|
India and Global Biosimilar Market
- Pioneer in the Global Biosimilars Market: India was the first country to approve a biosimilars product for Hepatitis B.
- Today, there are 98 approved biosimilars in India, with at least 50 in the market, the most in any country.
- Many India-made biosimilars have been approved in markets like the US.
- Rapid Growth of the Indian Biosimilars Market: The Indian biosimilars market was valued at $349 million in 2022 and is estimated to expand at a growth rate of 25.2 per cent per annum from 2022 to 2030 to reach $2,108 million by 2030.
- Expanding Scope: By 2030, biologic products worth some $170 billion will lose patent protection.
- This will open a window of opportunity for Indian biopharma to launch more biosimilar products.
- Regulatory Framework in India: In India, biopharmaceuticals and generic medicines are classified as drugs and are regulated by the Central Drugs Standard Control Organization (CDSCO) under the Drugs and Cosmetics Act, 1940.
Check Out UPSC CSE Books From PW Store
Intellectual Property and Patents in Pharmaceuticals
- Intellectual Property Rights: Intellectual property (IP) rights, particularly patents, incentivize innovation by granting inventors exclusive rights for a limited duration, typically 20 years from the filing date.
- This exclusivity allows pharmaceutical companies to recover substantial costs associated with drug development, including research, clinical trials, and regulatory approval.
- Patent evergreening:
- It refers to the practice of extending the life of a patent and patent protections beyond its original term.
- The Pharma Companies resort to Evergreening practices when a patent’s life is coming to an end by making minor modifications, which allow them to block and delay entry of biosimilars, thus keeping prices high.
- For example, Novartis sought multiple patents for imatinib, making modifications to the salt form and dosage to extend market exclusivity.
- Monopolistic Control: It can lead to monopolistic control over a product. By extending patent protection, companies can dominate the market, limiting competition and consumer choices.
|
Challenges Faced By India With Respect to Biosimilars
- Patent Evergreening: Patent evergreening is one of the significant barriers faced by Indian biosimilar manufacturers.
- Example: The multinational Roche extended the exclusivity of trastuzumab (Herceptin), a biologic used to treat breast cancer, by introducing a subcutaneous version just as the original patent was expiring.
- Regulatory Challenges: Biosimilars, unlike generic drugs, are derived from living organisms and require rigorous clinical trials to demonstrate similarity in quality, safety, and efficacy.
- The complexity of biologics leads to inherent variability, making approval and market entry more challenging.
- Example: The biosimilar of trastuzumab (used in breast cancer treatment) took several years to gain approval, affecting its timely entry into the market.
- Limited Testing Facilities: India currently faces a significant challenge with limited testing facilities capable of conducting the extensive bio-similarity assessments required for biosimilars.
- Adoption Hesitancy: There is often a lack of awareness among healthcare professionals and patients regarding the safety and efficacy of biosimilars, leading to hesitancy in adoption.
- This lack of understanding can limit patient access to potentially cost-effective treatment options.
- Market Competition: Intense competition from established global players in the biosimilars market can make it challenging for Indian manufacturers to secure market share.
- India holds only a 3% share of the global biosimilar market.
- Intellectual Property Challenges: Navigating the complexities of intellectual property laws and potential litigation can be daunting for Indian biosimilar companies.
- Example: Reliance Life Sciences (RLS) faced legal action from Roche regarding its biosimilar trastuzumab, used for treating breast cancer.
- The Delhi High Court in 2015 expressed concerns about Roche’s motives, suggesting the lawsuit aimed to stifle competition.
Check Out UPSC NCERT Textbooks From PW Store
Patent Regime in India
- In India, patents are governed by the Patents Act, 1970. This framework establishes the criteria for granting patents, which include:
- Patentable Subject Matter: Patents can be granted for new inventions, including processes or products that meet the criteria of novelty, inventive step, and industrial application.
- Exclusions: Certain exclusions apply, such as discoveries of mere scientific principles and methods of agriculture.
- The standard duration of a patent is 20 years from the date of filing.
- Evergreening Restrictions: Section 3(d) of the Patents Act aims to prevent evergreening by rejecting patents for minor modifications lacking significant therapeutic efficacy.
- Compulsory Licensing: It is a legal mechanism that allows a government to permit the production of a patented product without the patent owner’s consent, typically during emergencies or for life-threatening diseases.
- Legally supported by the TRIPS agreement, India has included this provision in Chapter XVI of its Patents Act, 1970, to ensure access to essential medicines.
- Regulating Authority: The Office of the Controller General of Patents, Designs and TradeMarks (CGPDTM) generally known as the Indian Patent Office, is an agency under the Department for Promotion of Industry and Internal Trade which administers the Indian law of Patents, Designs and TradeMarks.
- Amendments to the Patent Act: The Indian Patent Act of 1970 was amended to align with the Trade-Related Aspects of Intellectual Property Rights (TRIPS) Agreement of the World Trade Organization (WTO).
- The Amended Indian Patent Act of 2005 introduced provisions for granting patents specifically for pharmaceutical products.
|
Initiatives by the Government for India’s Biopharmaceutical Development
- National Biopharma Mission
- Initiative Overview: Under the Make in India initiative, the National Biopharma Mission (NBM) has been launched as an industry-academia collaborative mission, managed by the Biotechnology Industry Research Assistance Council (BIRAC).
- Funding and Scope: This $250 million mission is co-funded by the World Bank and aims to accelerate the development of biopharmaceuticals.
- Support for Organisations: The NBM supports nearly 150 organisations and 300 micro, small, and medium enterprises (MSMEs) in the biopharmaceutical sector.
- India’s Patent Legislation: Particularly Section 3(d) of the Patents Act, 1970, aims to prevent “evergreening” by rejecting patents for small innovations that lack substantive improvement.
-
- Example: In Novartis vs Union of India, the patent application of Novartis for the cancer drug Glivec (imatinib), used to treat leukaemia, was rejected as it did not show significant technical advancement.
- This set a strong precedent against evergreening practices.
- Section 3(e) of the Act restricts patenting mixtures of known compounds unless a synergistic effect is proven, and Section 3(i) prevents patents on treatment methods.
- Guidelines on Similar Biologics: Biosimilars are also known as Similar Biologics.
- The Guidelines on Similar Biologics are a comprehensive policy document outlining the regulatory pathway for approving similar biologics in India. It is developed collaboratively by the Central Drugs Standard Control Organization (CDSCO) and the Department of Biotechnology (DBT).
Way Forward
- Strengthen Patent Laws: Reinforce Section 3(d) of India’s Patents Act, which prevents patents for minor modifications.
- Encourage Compulsory Licensing: In cases of high public health impact, leverage compulsory licensing provisions to make essential biosimilars more accessible and reduce monopolistic control.
- Expand Clinical Trial Capabilities: Increase investment in domestic clinical trial infrastructure.
- Providing grants and incentives for companies to conduct biosimilar trials could make these trials more feasible and accessible.
- Re-establishment of Intellectual Property Appellate Board (IPAB): for early disposal of patent dispute cases.
- The Intellectual Property Appellate Board (IPAB) was abolished in April 2021 with the passing of the Tribunals Reforms (Rationalization and Conditions of Service) Ordinance, 2021.
- Implement Quality Management Systems: Standardise production protocols to ensure consistent biosimilar quality.
- The National Institute of Biologicals is an autonomous institute under India’s Ministry of Health and Family Welfare for theQuality Control of Biologicals.
- It can help set up quality control standards and monitoring.
- Encourage Venture Capital in Biopharma: Implement tax breaks or risk-sharing mechanisms to attract more private investment in the biosimilar sector, especially targeting smaller players with high growth potential.
- Advocate for Fair Intellectual Policies (IP): Through forums like World Trade Organization (WTO) and World Intellectual Property Organization (WIPO), India could advocate for fairer global IP practices, reducing litigation risks for biosimilars.
- Learning from Global Experiences: The European Medicines Agency’s 2005 biosimilar guidelines offer a streamlined approval process, resulting in substantial Biosimilar adoption in Germany, the UK, and Nordic countries, which has led to significant cost savings.
- In the United States, a legal framework for approving biosimilars was established in 2009, via the Biologics Price Competition and Innovation Act of 2009 (BPCI Act). The BPCI Act establishes an abbreviated approval pathway for biosimilars.
Enroll now for UPSC Online Classes
Conclusion
India’s pharmaceutical and biotech industry requires strong government support and robust patent laws to successfully capture the biologics and biosimilars market.
![]() 29 Oct 2024
29 Oct 2024

