In a world-first, US researchers successfully used CRISPR-based gene editing to treat a baby named KJ with CPS-1 deficiency, a life-threatening genetic disorder.
About CPS-1 Deficiency
- Carbamoyl Phosphate Synthetase 1 (CPS-1) deficiency is a rare urea cycle disorder that impairs the liver’s ability to eliminate ammonia, with nearly 50% infant mortality.
- Symptoms: The following signs are seen in patients namely: lethargy and respiratory distress due to ammonia build-up in the body.
- Limitations of Conventional Treatment: Liver transplant is often the only long-term treatment, but infants typically aren’t ready for the procedure early in life.
- Therapeutic Breakthrough: Doctors used customised CRISPR-based gene-editing therapy called “k-abe” to correct the mutation in KJ’s genome.
- Targeted Delivery: The gene-editing tool was encapsulated in lipid nanoparticles for safe delivery to the liver.
- Outcomes: After three infusions, the baby could tolerate protein, needed lower medication doses, and began gaining weight without showing any severe side effects.
- FDA Approval: The U.S. FDA granted emergency approval for this personalized therapy, highlighting the growing acceptance of gene-editing in rare disease management.
Significance of the Success
- Clinical success of In-vivo Editing : This was the first individualized in-vivo gene-editing treatment conducted directly inside a human body.
- Customised Therapy: It paves the way for targeted treatments of thousands of rare genetic diseases where organ transplant or lifelong drug use are currently the only options.
- Precise Treatment: The use of lipid nanoparticles ensured safe and precise delivery to the liver without triggering immune complications.
What is Gene Editing Therapy?
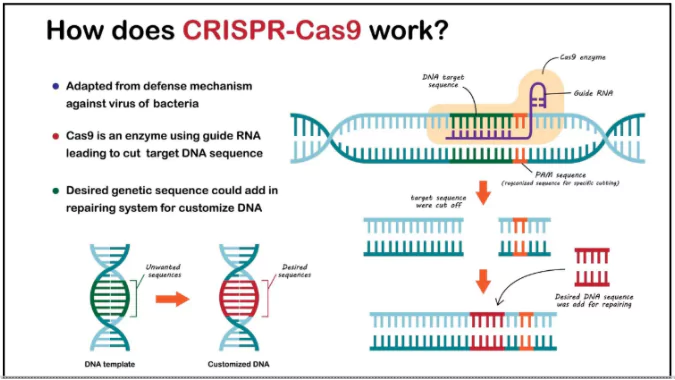
- Gene editing therapy is a form of treatment where DNA is altered to correct defective genes responsible for disease development.
- It differs from gene therapy, which generally adds a normal gene without altering the existing one.
- Tool Used: The most common tool is CRISPR-Cas9, which acts as molecular scissors to cut and replace faulty DNA sequences.
Types of Gene Therapy
Types based on Cell Targeted
| Aspect |
Somatic Gene Therapy |
Germline Gene Therapy |
| Target Cells |
Somatic gene therapy targets the body’s non-reproductive cells, such as liver or blood cells. |
Germline gene therapy targets the reproductive (germ) cells, including sperm or egg cells. |
| Heritability |
The genetic changes made in somatic cells are not passed onto future generations. |
The genetic modifications are heritable and can be transmitted to offspring. |
| Therapeutic Scope |
It is used to treat existing patients suffering from genetic disorders or diseases. |
It aims to prevent genetic disorders from being inherited by future generations. |
| Ethical Acceptability |
Somatic gene therapy is widely accepted and used in clinical practice globally. |
Germline gene therapy is ethically controversial and is currently restricted or banned in many countries. |
| Risk Level |
It has a lower risk of unintended long-term consequences, as changes affect only the treated individual. |
It involves a higher risk, as unintended genetic changes can affect future generations permanently. |
| Examples |
Treatment of sickle cell anemia, hemophilia, and leukemia using gene addition. |
Potential future correction of Tay-Sachs, cystic fibrosis, or Huntington’s disease at embryo stage. |
Types Based on site of Procedure: In-Vivo and Ex-Vivo Therapy
| Aspect |
In-Vivo Gene Therapy |
Ex-Vivo Gene Therapy |
| Definition |
Gene editing is performed directly inside the patient’s body. |
Gene editing is performed outside the body on cells extracted from the patient. |
| Procedure |
Therapeutic genes or gene-editing tools are delivered to targeted tissues in the body. |
Cells are modified in a laboratory and then reintroduced into the patient’s body. |
| Time and Complexity |
It reduces the overall time and complexity by avoiding cell extraction and reinfusion. |
It is more complex due to the need for cell isolation, culture, and reinfusion. |
| Delivery Method |
Uses viral vectors or nanoparticles (e.g., lipids) for targeted tissue delivery. |
Uses electroporation or viral vectors in controlled lab settings for gene insertion. |
| Tissue Access |
Best suited for tissues that are hard to access or modify outside the body. |
More suitable for blood or immune cells that are easily extracted and reinfused. |
| Example |
CRISPR was used directly in the liver to treat CPS-1 deficiency in infant KJ. |
CAR-T cell therapy for cancer involves ex-vivo modification of T-cells. |
Applications
- Current Use: Treatment for sickle cell anemia, cystic fibrosis, hemophilia, leukemia, and now CPS-1 deficiency.
India’s Preparedness in Gene Therapies
- Policy Framework: India has released Draft Guidelines on Gene Therapy Product Development and Clinical Trials.
- Infrastructure:Emerging biotechnology hubs such as the CSIR – Institute of Genomics and Integrative Biology (CSIR-IGIB), the International Centre for Genetic Engineering and Biotechnology (ICGEB), and National Institutes of Health (NIH)-funded laboratories are conducting cutting-edge research in areas like gene editing, molecular diagnostics, and therapeutic innovation.
- Way forward: India needs to expand public investment, accelerate trial approvals, and develop affordable indigenous gene therapies for rare diseases.
- The current Outcome of Genome India Project is a welcome step in this direction.
|
- Potential Use: Could treat diseases like Alzheimer’s, Parkinson’s, heart disease, and other metabolic or neurodegenerative disorders.
- Emerging Use: In cancer immunotherapy, where edited cells target and kill tumor cells.
Ethical and Regulatory Roadblocks
- Ethical Concerns: Gene editing poses risks of unintended genetic mutations, long-term health consequences, and controversies around germline modifications that affect future generations.
- Informed consent becomes complex, especially in infants.
- Chances of Misuse: There’s growing fear around the potential misuse of technology for non-therapeutic enhancements, such as creating “designer babies” with selected traits, raising socio-ethical and justice concerns.
- Regulatory Oversight: The field demands strict regulatory frameworks to ensure safety, efficacy, and accountability, especially when used in vulnerable populations like newborns.
- Global Divergence: While nations like the US and China lead in trials and approvals, many countries remain cautious, citing lack of long-term safety data and unresolved ethical questions.
- Cost and Accessibility: Gene therapies are highly expensive, limiting access to affluent populations and widening healthcare inequality. Ensuring affordability remains a significant policy challenge.
Conclusion
The success of gene-editing therapy in saving an infant with CPS-1 deficiency marks a transformative leap in precision medicine. With ethical caution and regulatory clarity, such personalized genomic interventions can revolutionize the future of healthcare, including in countries like India.
![]() 19 May 2025
19 May 2025

