Recently, India’s first-in-human gene therapy trial for Haemophilia A was conducted at BRIC-inStem
About Haemophilia A
- Haemophilia A is a rare genetic bleeding disorder caused by a deficiency of clotting protein Factor VIII, due to mutations in the F8 gene on the X chromosome.
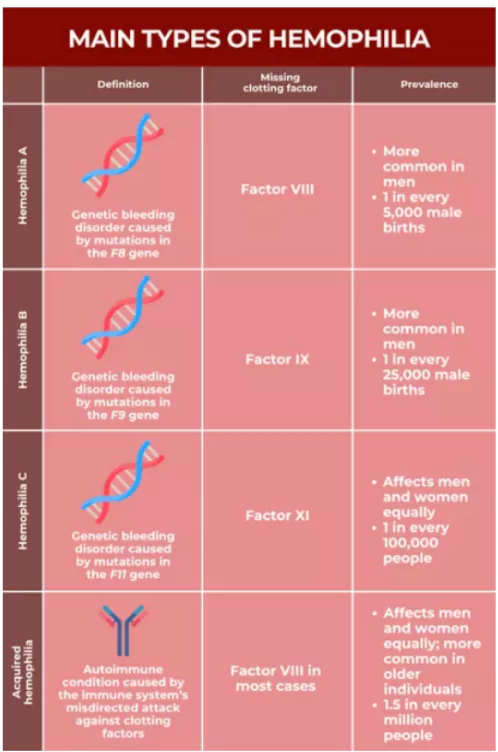 Vulnerability: It primarily affects males; females can be carriers and may occasionally show symptoms if both X chromosomes carry the mutation.
Vulnerability: It primarily affects males; females can be carriers and may occasionally show symptoms if both X chromosomes carry the mutation.- Symptoms: It includes excessive bleeding, easy bruising, and joint pain due to internal bleeding.
- Treatment: Traditionally, treatment involved frequent intravenous infusions of clotting factors, but there is no permanent cure without advanced therapies.
About Gene Therapy for Haemophilia
- Gene therapy offers a potential one-time cure by delivering a functional copy of the F8 gene into the patient’s liver cells using a modified adenovirus vector.
- The therapy instructs the liver to produce Factor VIII internally, thereby correcting the clotting deficiency from the source.
- The first commercial gene therapy, Roctavian, was approved by the U.S. FDA in 2023, setting a precedent for similar global trials, including India’s.
- India’s clinical trials mark a turning point in making gene therapy accessible and affordable for the Indian population.
This breakthrough positions India at the forefront of next-generation biomedical innovation, with gene therapy emerging as a pillar of future healthcare and economic growth.
Key Achievements of India In Biotechnology
- India’s biotech sector has expanded 16-fold in a decade, reaching $165.7 billion in 2024, with a target of $300 billion by 2030.
- The newly launched BIO-E3 Policy focuses on enhancing the economy, employment, and environment through biotechnology.
- Establishment of the Biotechnology Research and Innovation Council (BRIC) by the Department of Biotechnology ( Ministry of Science and technology) unified 14 autonomous institutions, accelerating innovation and collaboration.
- Biotechnology Industry Research Assistance Council’s Institute for Stem Cell Science and Regenerative Medicine (BRIC-inStem), Bengaluru, is leading translational research in regenerative medicine, with trials in collaboration with CMC Vellore and other top institutes.
- Infrastructure such as Biosafety Level III labs and the Centre for Research Application and Training in Embryology (CReATE) enhance preparedness and maternal health research.
|
![]() 25 Apr 2025
25 Apr 2025

 Vulnerability: It primarily affects males; females can be carriers and may occasionally show symptoms if both X chromosomes carry the mutation.
Vulnerability: It primarily affects males; females can be carriers and may occasionally show symptoms if both X chromosomes carry the mutation.