Hundred million tonnes of nitrogen are now removed from the atmosphere and converted into fertilizer via the Haber-Bosch process, adding 165 million tonnes of reactive nitrogen to the Soil.
Haber-Bosch process
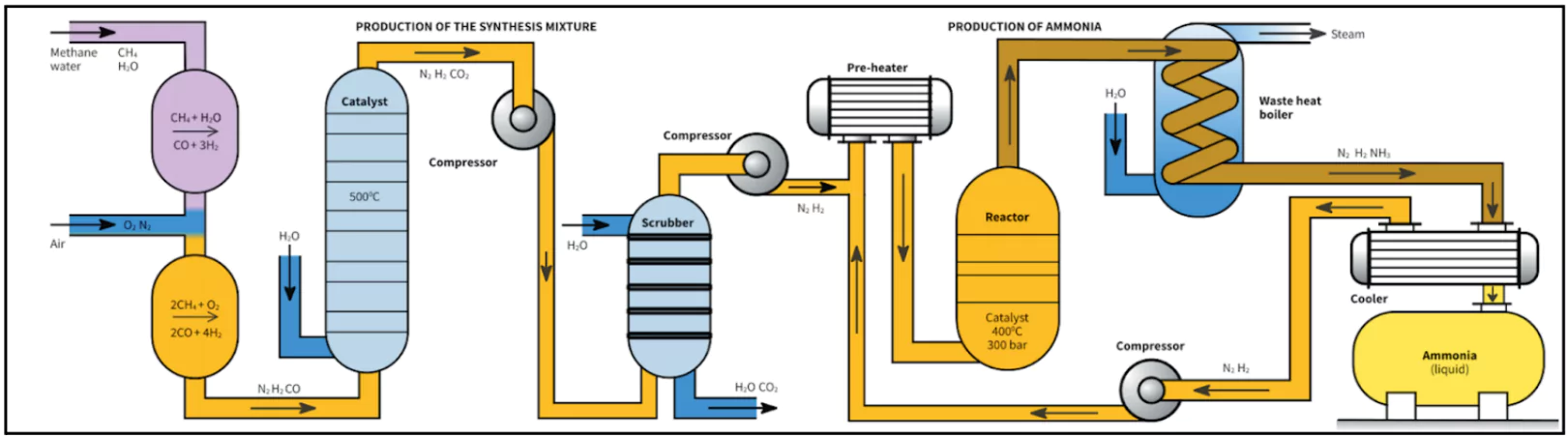
The Haber-Bosch process involves the transformation of Atmospheric nitrogen (N2) into ammonia (NH3) via a reaction with hydrogen (H2).
- This process employs a metal catalyst (iron) and operates under high temperatures and pressures.
Key Impact of the Haber-Bosch Process
- Increased Food Production: By enabling the mass production of ammonia, a key ingredient in synthetic fertilisers, the Haber-Bosch process allowed for a dramatic increase in crop yields, making it possible to feed the rapidly growing global population.
- Synthetic Nitrogen Fertilisers: The process removed 100 million tonnes of nitrogen from the atmosphere annually, converting it into 165 million tonnes of reactive nitrogen fertilisers. This surpasses the nitrogen added naturally through biological processes.
- Population Growth: It is estimated that one-third of the global population today depends on food grown using nitrogen fertilisers made possible by this process. Without it, widespread famine and malnutrition would be far more common.
- Impact on Ecosystems: Excess nitrogen from fertilisers has caused environmental damage, including acid rain, soil degradation, waterway pollution, and the depletion of oxygen in aquatic systems due to eutrophication.
Enroll now for UPSC Online Classes
About Nitrogen
- Nitrogen in the air is mostly in the form of N2 ,where two nitrogen atoms join together.
- They share three pairs of electrons to form a triple bond making the molecule nearly unbreakable.
- Energy required to break the nitrogen triple bond is so high (946 kJ/mol) that molecular nitrogen is nearly inert.
- Nitrates: Molecules of oxygen and nitrogen which is abundant in the earth’s atmosphere.
- On reaction, atomic nitrogen can form ionic nitrides which are also known as reactive nitrogen such as ammonia (NH3), Ammonium (NH4+) or nitrates (NO3-).
- Role of Reactive Nitrogen: Plants need these types of nitrogen to synthesise enzymes, proteins, and amino acids.
- Legumes can produce nitrogen independently
- But important food crops such as rice, wheat, corn, and potatoes and common fruits and vegetables draw nitrogen from the soil.
- As the human population multiplies, Nitrogen in agricultural soil depletes faster, needing fertilisers to compensate.
- Humans and Animals: Need nine pre-made nitrogen rich amino acids from plants.
- Nitrogen makes up approximately 2.6% of the human body.
How is nitrogen available in Nature?
- Lightning
- Only lightning has enough energy to destroy the N triple bond. In a lightning bolt, nitrogen in the air combines with oxygen to generate nitrogen oxides such as NO and NO2.
- They can then combine with water vapor to create nitric and nitrous acids (HNO3 and HNO2, respectively).
- Reactive nitrogen-rich droplets: Fertilize farmlands, woods, and grasslands when it rains.
- This pathway is estimated to replenish soil by around 10 kg of nitrogen per acre per year.
- Metabolic Process
- Carried out by Azotobacter bacteria which create Reactive nitrogen.
- Some microorganisms such as Rhizobia have developed symbiotic relationships with legume plants (clover, peas, beans, alfalfa, and acacia) to provide reactive nitrogen in exchange for nutrition.
- Azolla, a species of aquatic fern with a symbiotic association with the cyanobacterium Anabaena azollae which can absorb and convert nitrogen from the air to reactive nitrogen, so dried and decaying Azolla is an effective fertilizer for farmland
Check Out UPSC NCERT Textbooks From PW Store
Nitrogen cycle
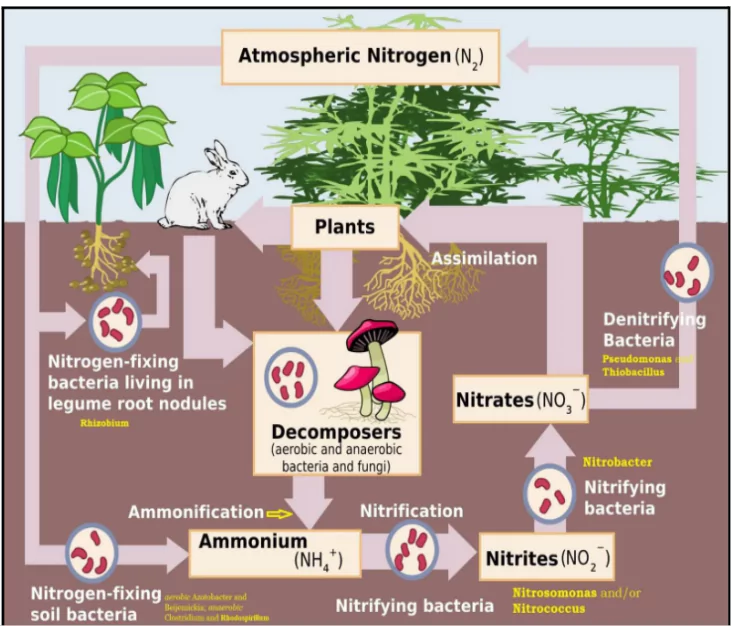
- Nitrogen Fixation: Atmospheric nitrogen (N₂) is converted into ammonia (NH₃) or ammonium ions (NH₄⁺) by nitrogen-fixing bacteria, which can be found in soil or in symbiotic relationships with plants (like legumes).This step makes nitrogen accessible to living organisms.
- Nitrification:
-
- Fist stage: Ammonia is oxidized to nitrite (NO₂⁻) by bacteria such as Nitrosomonas.
- Second Stage: Nitrite is further oxidized to nitrate (NO₃⁻) by bacteria like Nitrobacter. Nitrate is a form that plants can readily uptake.
- Ammonification (or Mineralization):Decomposition of organic matter (dead plants and animals, as well as animal waste) releases ammonia back into the soil. This step recycles nitrogen back into the ecosystem.
- Denitrification: Nitrates are converted back into nitrogen gas (N₂) by denitrifying bacteria (such as Pseudomonas) and released into the atmosphere.
- This step completes the nitrogen cycle by returning nitrogen to the atmosphere, helping to regulate its levels.
![]() 15 Oct 2024
15 Oct 2024

