After two years of political negotiations, 194 World Health Organization (WHO) member states failed to finalise a historic Pandemic Agreement
- It was an international treaty designed to fortify global pandemic preparedness, implement mechanisms for prevention of the same, and reduce unconscionable inequities that were painfully obvious during the COVID-19 pandemic.
About WHO Pandemic Treaty
- Existing Mechanism: WHO already has binding rules known as the International Health Regulations (2005) which set out countries’ obligations where public health events have the potential to cross national borders.
- These include advising the WHO immediately of a health emergency and measures on trade and travel.
- Background of WHO Pandemic Treaty: In March 2021, an extraordinary call for a pandemic treaty was issued by 25 heads of government and international agencies, marking a pivotal moment in global health governance.
Enroll now for UPSC Online Course
International Health Regulations (2005) (IHR)
- Objective: It provides an overarching legal framework that defines countries’ rights and obligations in handling public health events and emergencies that have the potential to cross borders.
- Legally Binding: The IHR is an instrument of international law that is legally-binding on 196 countries, including the 194 WHO Member States.
- Origin: The IHR grew out of the response to deadly epidemics that once overran Europe. They create rights and obligations for countries, including the requirement to report public health events.
- About: The Regulations also outline the criteria to determine whether or not a particular event constitutes a “public health emergency of international concern”.
- IHR introduces important safeguards to protect the rights of travellers and other persons in relation to the treatment of personal data, informed consent and non-discrimination in the application of health measures under the Regulations.
- Limitation: Adopted after the 2002/3 SARS outbreak, these regulations are still seen as functional for regional epidemics such as Ebola but inadequate for a global pandemic.
|
Why did WHO’s Member States decide to create an accord for pandemic preparedness and response?
- Limitations of International Health Regulations: The existing International Health Regulations are already legally binding. However, they failed to prevent unjust travel or trade restrictions, and hoarding of vaccines and other medical countermeasures during the COVID-19 pandemic.
- Draft & Negotiation: In light of the impact of the COVID-19 pandemic, WHO’s 194 Member States established a process to draft and negotiate a new convention, agreement, or other international instrument on pandemic preparedness and response.
- Need: This was driven by the need to ensure communities, governments, and all sectors of society are better prepared and protected, in order to prevent and respond to future pandemics.
- The great loss of human life, disruption to households and societies at large, and impact on development are some factors cited by governments to support the need for lasting action to prevent a repeat of such crises.
- Balancing: To ensure equity in both access to the tools needed to prevent pandemics (including technologies like vaccines, personal protective equipment, information and expertise) and access to health care for all people.
India’s Pandemic Management System
It encompasses a range of strategies and frameworks designed to prepare for, respond to, and mitigate the effects of pandemics. This system involves multi-level coordination among various government agencies, healthcare institutions, and international organizations.
Policy Framework
- National Health Policy: Provides broad guidelines for healthcare planning, including pandemic preparedness.
- Disaster Management Act (2005): Establishes the framework for managing emergencies, including pandemics.
- National Action Plan for COVID-19: Developed specifically for the COVID-19 pandemic to outline strategies and interventions.
Institutional Mechanisms
- Ministry of Health and Family Welfare (MoHFW): Responsible for formulating policies and overseeing health services.
- National Disaster Management Authority (NDMA): Coordinates disaster response and provides guidelines for emergency management.
- Indian Council of Medical Research (ICMR): Conducts research and provides guidance on disease management and vaccine development.
Surveillance and Data Management
- Integrated Disease Surveillance Programme (IDSP): Monitors and tracks disease outbreaks and provides real-time data.
- National Centre for Disease Control (NCDC): Analyzes data, conducts surveillance, and provides technical support for managing diseases.
- Aarogya Setu App: A contact-tracing and health status app launched during the COVID-19 pandemic to track exposure and provide health updates.
Public Health Interventions
- Vaccination Campaigns: Mass immunization drives for various diseases, including COVID-19
- Quarantine and Isolation: Measures to isolate affected individuals and prevent the spread of infectious diseases.
- Health Guidelines: Issuance of guidelines for social distancing, hygiene practices, and travel restrictions during outbreaks.
|
Key Developments at the 77th World Health Assembly (WHA)
Under the theme “All for Health, Health for All,” discussions were centered around the World Health Organization (WHO) new strategy for global health 2025-2028.
Check Out UPSC CSE Books From PW Store
Few Notable Changes proposed are:
- Amendments to International Health Regulations (IHR) 2005:
-
- Key Changes: The IHR amendments aim to enhance the ability of countries to prepare for and respond to Public Health Emergencies of International Concern (PHEIC) and introduce a new category for urgent international response — a Pandemic Emergency (PE).
- AIM: The amendments aim to ensure equitable access to health products during health emergencies and to mobilise financial resources to support developing countries in building and maintaining core health system capacities required under the IHR.
- National IHR: It mandates the creation of a National IHR Authority for better coordination.
- Pandemic Emergency: It include the definition of a Pandemic Emergency, which represents the new highest level of alarm contained within the IHR and available for use by the WHO Director-General.
- AIM: A Pandemic Emergency aims to trigger a more effective international collaboration in response to events that are at risk of becoming, or have become, a pandemic.
Pandemic Emergency
Each and all of the six following criteria must be met for an “event”) to be determined a “Pandemic Emergency”.
- It must be a Public Health Emergency of International Concern (PHEIC).
- A PHEIC means an extraordinary event which is determined
- To constitute a public health risk to other States through the international spread of disease
- To potentially require a coordinated international response
- Being of communicable disease nature
- Having or at risk of having wide demographical
- Exceeding, or is at high risk of exceeding, the capacity of health systems
- Causing, or is at high risk of causing, substantial social and/or economic disruption etc
- Requires rapid, equitable and enhanced coordinated international action etc.
|
- INB: The 77th WHA extended the mandate of the Pandemic Treaty negotiating body, namely, the intergovernmental negotiating body (INB), stipulating that the proposed WHO Pandemic Agreement must be completed as soon as possible.
- Outcome to be discussed in 78th WHA: The outcome should be submitted for consideration at the 78th World Health Assembly in May 2025, or earlier if possible, at a Special Session of the World Health Assembly in 2024.
Intergovernmental Negotiating Body (INB)
- Establishment: In December 2021, at its second-ever special session, the World Health Assembly established an intergovernmental negotiating body (INB) to draft and negotiate a convention, agreement or other international instrument under the Constitution of the World Health Organization to strengthen pandemic prevention, preparedness and response.
- The INB’s work is based on the principles of inclusiveness, transparency, efficiency, Member State leadership and consensus.
|
Contentious Issues in the Pandemic Agreement
Three key contentious issues in the latest draft of the Pandemic Agreement remain significant obstacles to its adoption:
- Pathogen Access and Benefit Sharing (PABS) System:
- AIM: To ensure that genetic resources and pathogen samples shared from developing countries (which are the most likely sources for such pathogens), are reciprocated with corresponding benefits such as vaccines and diagnostics that result from research and development on samples and data provided from the Global South.
- Article 12: Pathogen Access and Benefit Sharing (PABS) system in Article 12, often seen as the “heart” of the agreement.
- Latest Proposal – Donating a portion: Manufacturers of vaccines and diagnostics primarily based in wealthy countries which are using genetic information from pathogens in low- and middle-income countries, would commit to donating a portion of their products to WHO for global distribution based on the principles of need and effectiveness.
- Contention:
- Low- and middle-income countries (LMICs) seek at least 20% of shared pandemic products.
- High-income countries propose 20% as the maximum limit; some do not agree to even this.
- Technology Transfer and Intellectual Property:
- Articles 10 and 11: Division over governance of production and technology transfer, and its implications on intellectual property, outlined in Articles 10 and 11
- Challenges:
- Reason for Vaccine Inequity: Intellectual property protections, export restrictions, and manufacturing limitations led to vaccine inequity during COVID-19.
- Strong provisions for technology transfers and local production needed for self-sufficiency of LMICs.
- Lack of consensus:
- Technology Transfer Provision: The central issue is the conditions for technology transfer to “facilitate sustainable and geographically diversified production” through mechanisms such as product information sharing and use of WTO- Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS) flexibilities such as compulsory licensing.
- No Consensus: There remains a lack of consensus on the transfer of ‘know-how’ and the binding nature of these transfers.
- High-income countries: They advocate for Voluntary and Mutually Agreed Terms (VMAT), but the use of VMAT language could discourage countries, particularly LMICs, from adopting mandatory approaches recognised under the TRIPS Agreement.
- Disagreement on Peace Clause: Peace clause requires member states to respect the use of the TRIPS flexibilities and not exercise any direct or indirect pressure to discourage the use of such flexibilities.
Check Out UPSC NCERT Textbooks From PW Store
What is TRIPS?
- About: TRIPS stands for “Trade-Related Aspects of Intellectual Property Rights.” It is an international agreement that is part of the World Trade Organization (WTO) framework.
- TRIPS was established to establish consistent and standardized rules for intellectual property (IP) rights on a global scale, ensuring that member countries have a common framework for protecting various forms of intellectual property, including patents, copyrights, trademarks, and trade secrets.
Relaxation Favoring TRIPS
- “Relaxation Favoring TRIPS” refers to instances where certain flexibilities or exceptions are introduced in the implementation of the Trade-Related Aspects of Intellectual Property Rights (TRIPS) agreement to address specific needs, especially in the context of public health and development.
- These relaxations aim to strike a balance between intellectual property protection and other crucial societal interests.
- Some notable examples of relaxation measures within TRIPS include:
- Compulsory Licensing: This allows governments to grant licenses for the production of patented products or processes without the patent holder’s consent. It is often invoked to ensure access to essential medicines and promote public health, particularly during emergencies like epidemics.
- Parallel Importation: This allows the importation of patented goods from one country to another without the patent holder’s consent. It can help increase competition and lower prices for patented products, benefiting consumers.
- Bolar Exemption: This permits the use of patented inventions for research and experimentation before the patent expires. It is significant in the pharmaceutical sector for conducting clinical trials and seeking regulatory approvals.
- Non-Exclusive Licensing: Governments can promote technology transfer by granting non-exclusive licenses for certain patents, encouraging local production and innovation.
- Protection of Public Health: TRIPS acknowledges the importance of safeguarding public health. In cases of national emergencies or other circumstances, countries can take measures to protect public health and ensure access to affordable medicines.
- Exemption for Least Developed Countries (LDCs): LDCs are given an extended transition period for implementing certain TRIPS provisions, recognizing their unique developmental challenges.
|
- One Health Approach:
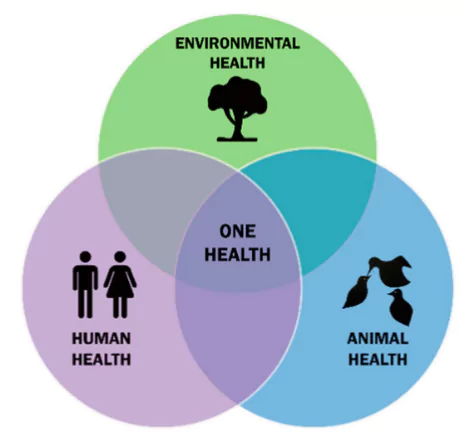 One Health: It is an integrated, unifying approach that aims to sustainably balance and optimize the health of people, animals and ecosystems.
One Health: It is an integrated, unifying approach that aims to sustainably balance and optimize the health of people, animals and ecosystems.- Draft Agreement: It requires member states to adopt a pandemic preparedness and surveillance approach that recognises the interconnection between the health of people, animals, and the environment
- It promotes a coherent, integrated, coordinated, and collaborative effort among all relevant organisations, sectors, and actors, as appropriate.
- Challenges:
- High-income countries: Particularly the European Union, strongly supports One Health.
- LMICs: They view it as an unfunded mandate that imposes an additional burden on their already strained resources
Other Concerns Related to the WHO Pandemic Agreement
- Enforcement and Compliance: The enduring obstacle in international law is its enforcement
- Lack of Robust Mechanism: International law often lacks robust enforcement mechanisms, leading to concerns about real accountability.
- Monitoring and Evaluation: The effectiveness of the proposed Conference of Parties (COP) in ensuring compliance remains uncertain.
- Equity and Solidarity
- Global North vs. Global South: Geopolitical discord and competing interests between higher- and lower-income countries hinder consensus.
- Historical Inequities: Past experiences, like the COVID-19 pandemic, highlighted severe inequities in treatment access and vaccine distribution, raising skepticism about future commitments.
- National Sovereignty: Countries prioritize their sovereignty and may resist external interference or mandatory compliance measures imposed by international agreements.
- Financial Resources and Support: Ensuring equitable access to health products and supporting developing countries in building and maintaining health system capacities require significant financial resources.
- Sustainability: Long-term sustainability of the agreement’s provisions is a concern, particularly for low- and middle-income countries.
- Long-Term Access and Self-Sufficiency
- Dependency on High-Income Countries: Developing countries’ reliance on “charity” from wealthier nations for medical products is seen as unsustainable.
- Manufacturing Capacities: The need for diverse and geographically dispersed manufacturing capacities to ensure long-term access to medical products.
Way Forward
- Mutual Solidarity: Achieving global health security requires mutual solidarity and cooperation, recognizing that health threats do not respect borders. Countries must prioritize collective interests over individual gains.
- Diplomatic Efforts: Skilled diplomacy is essential to bridge gaps between countries’ interests and foster a cooperative spirit for the effective implementation of the Pandemic Agreement.
- Incentives and Support Mechanisms: Providing incentives for compliance, such as financial aid, technical assistance, and capacity-building support, can encourage adherence to the agreement.
- However, securing sufficient resources and equitable distribution of support poses difficulties.
- Strengthening International Institutions: Empowering international health institutions with greater authority and resources can enhance their capacity to enforce compliance and support member states in meeting their obligations.
- Strengthening Enforcement Mechanisms
- Robust Compliance Framework: Develop a more stringent compliance framework to ensure member states adhere to the treaty’s provisions.
- Regular Monitoring: Establish frequent and transparent monitoring mechanisms to track the progress of implementation.
- Penalties for Non-compliance: Introduce penalties for countries that fail to comply with the treaty to ensure accountability.
- Monitoring and Evaluation Systems: An inclusive, transparent, and effective monitoring and evaluation system is crucial for accountability.
- However, designing and implementing such a system that is acceptable to all member states remains a significant challenge.
- Capacity Building in Low- and Middle-Income Countries (LMICs):
- Technical Assistance: Provide technical assistance to LMICs to build their healthcare infrastructure and response capabilities.
- Training and Education: Implement training programs to enhance the skills of healthcare workers in LMICs.
- Infrastructure Development: Invest in healthcare infrastructure to ensure LMICs are better prepared for future pandemics.
- Conference of Parties (COP): The Pandemic Agreement proposes the establishment of a COP to monitor implementation and review compliance every five years.
- However, the effectiveness of this body depends on its authority, resources, and the willingness of member states to cooperate.
Enroll now for UPSC Online Classes
Conclusion
- A core aim of the Pandemic Agreement — beyond ensuring the immediate availability of medical products during emergencies — should be to promote long-term and sustainable access to these products by diversifying production and enhancing regional manufacturing capabilities
- The coming months of negotiations are crucial. This treaty is not just for the next pandemic but also serves as a blueprint for a more equitable and resilient global health system.
![]() 1 Aug 2024
1 Aug 2024

 One Health: It is an integrated, unifying approach that aims to sustainably balance and optimize the health of people, animals and ecosystems.
One Health: It is an integrated, unifying approach that aims to sustainably balance and optimize the health of people, animals and ecosystems.