Context:
Scientists at the University of Oxford U.K. have launched first-in-human Nipah Virus Vaccine trials for the Nipah virus disease.
Nipah Virus Vaccine: ChAdOx1 NipahB
- Nipah Virus Vaccine Name: ChAdOx1 NipahB vaccine.
- The vaccine uses the ChAdOx1 platform, the same that was used for the Oxford/AstraZeneca COVID-19 vaccine.
- Trail: It will consist of 51 people aged 18 to 55, for a period of the next 18 months, led by the Oxford Vaccine Group.
Viral Vector-based vaccines
- They use a harmless virus to smuggle the instructions for making antigens from the disease-causing virus into cells, triggering protective immunity against it.
- It differs from most conventional vaccines in that they don’t actually contain antigens, but rather use the body’s own cells to produce them.
- Mechanism: It uses a modified virus (the vector) to deliver genetic code for antigen, in the case of COVID-19 spike proteins found on the surface of the virus, into human cells.
- Vector infects cells and instructs them to make large amounts of antigen, which then trigger an immune response, the vaccine mimics what happens during natural infection with certain pathogens – especially viruses.
|
About Nipah Virus
- About: It belongs to a family of paramyxoviruses.
- First identified: In 1998 in Malaysia and Singapore in an outbreak among pig farmers.
- Carriers: Fruit bats of the family Pteropodidae acts as a natural host of the virus.
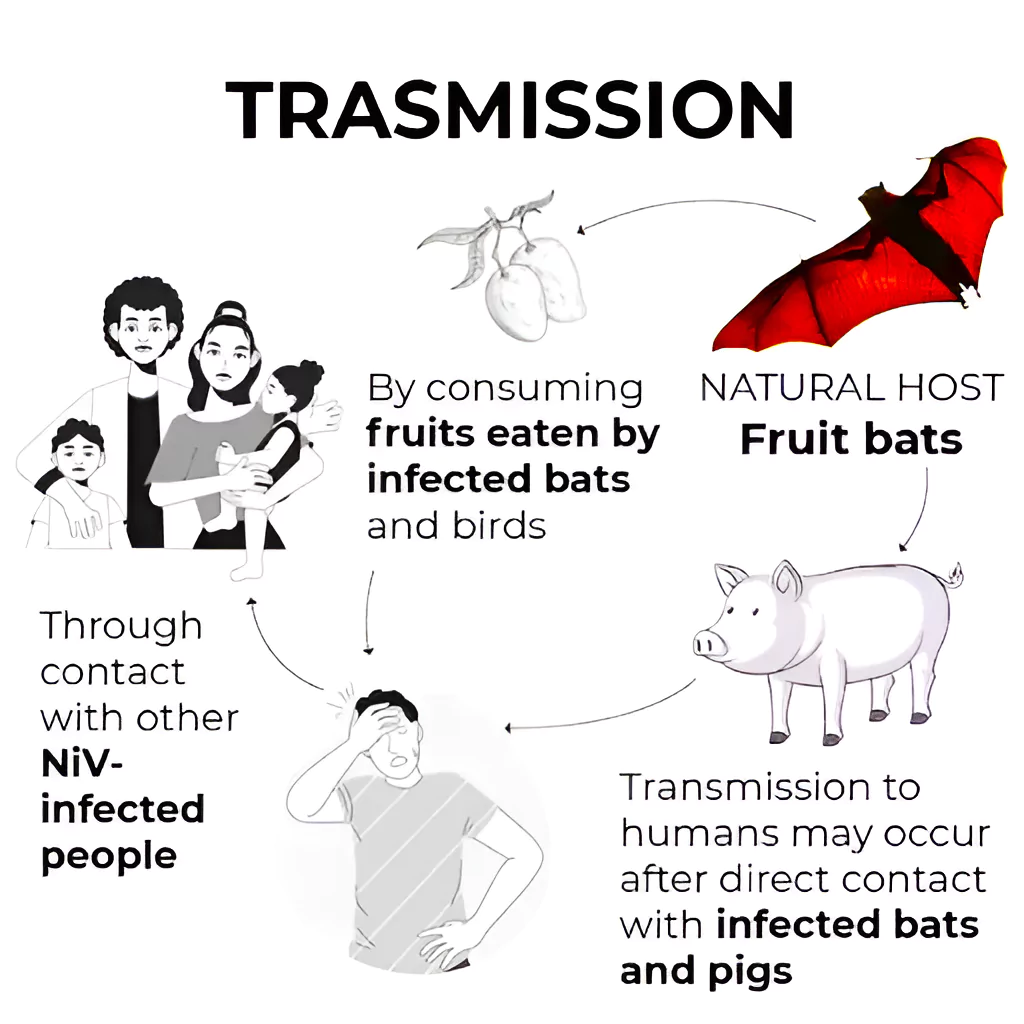
Transmission of Nipah Virus
- Outbreaks: Outbreaks have occurred in countries in Asia, including Singapore, Malaysia, Bangladesh and India with Kerala seeing annual outbreaks. Nature of Transmission:
- It is zoonotic in nature ( transmitted from animals to humans)
Priority Disease for WHO Research and Development Blueprint:
- About: It is a WHO tool distinguishing the diseases which pose the greatest public health risk due to their epidemic potential and whether there is sufficient countermeasures available.
- Objective: To ensure efforts under WHO’s R&D Blueprint are focused and productive, a list of diseases and pathogens are prioritized for R&D in public health emergency contexts.
- Examples: COVID-1, Ebola virus, Severe Acute Respiratory Syndrome (SARS), Nipah, Rift Valley fever, Zika and Disease X.
|
-
- Unprotected exposure to secretions or tissues of a sick animal (Eg: Malaysia)
- Consumption of fruits or fruit products (such as raw date palm juice) contaminated with urine or saliva from infected fruit bats was the most likely source of infection ( Example: cases in Kerala)
- Human-to-human transmission of Nipah virus has also been reported among family and caregivers of infected patients. (Example: Siliguri, India in 2001, 75% of cases occurred among hospital staff or visitors.)
- Infection: Human infections range from asymptomatic infection to acute respiratory infection (mild, severe), and fatal encephalitis progressing to coma within 24 to 48 hours.
- The incubation period (interval from infection to the onset of symptoms) is believed to range from 4 to 14 days.
- Diagnosis: The main tests used are real-time polymerase chain reaction (RT-PCR) from bodily fluids and antibody detection via enzyme-linked immunosorbent assay (ELISA).
- Treatment: There are currently no drugs or vaccines specific for Nipah virus infection.
- WHO Status: WHO has identified Nipah as a priority disease for the WHO Research and Development Blueprint saying it has pandemic potential.
Also Refer:
News Source: The Hindu
![]() 18 Jan 2024
18 Jan 2024
