Atoms of most elements are not able to exist independently. They form molecules and ions. These molecules or ions aggregate in large numbers to form the matter that we can see, feel or touch.
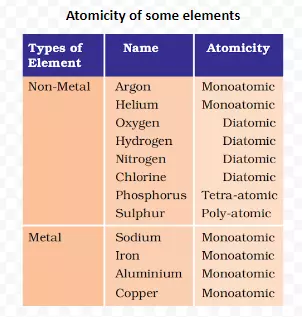
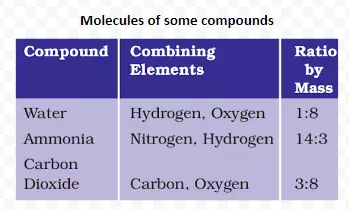
Meaning: Atoms of different elements join together in definite proportions to form molecules of compounds.
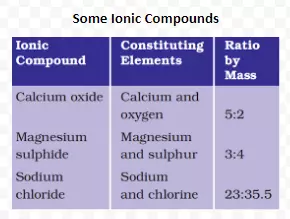
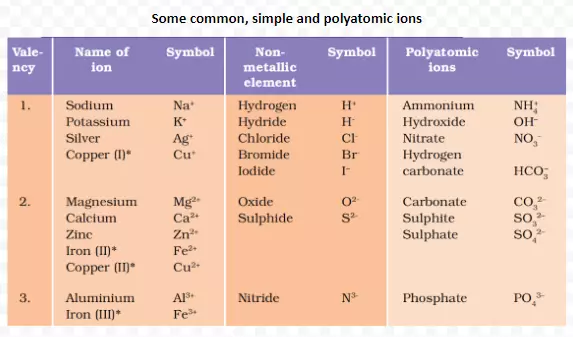
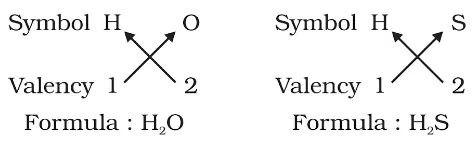
<div class="new-fform">
</div>
