![]() 20 Dec 2023
20 Dec 2023
When the nature and the identity of the initial substance have somewhat changed it is said to be a chemical change and whenever a chemical change occurs, a Chemical Reaction takes place. These Chemical Reactions can be represented as chemical equations.
Magnesium + Oxygen → Magnesium oxide
(Reactants) (Product)
Mg + O2 → MgO
Example: Zn + H2SO4 → ZnSO4 + H2
|
Number of atoms of different elements on both sides of the arrow |
||
| Element | Number of atoms in reactants (LHS) | Number of atoms in products (RHS) |
| Zn | 1 | 1 |
| S | 2 | 2 |
| H | 1 | 1 |
| O | 4 | 4 |
Fe + H2O → Fe3O4 + H2
|
Number of atoms of different elements present in the unbalanced equation |
||
| Element | Number of atoms in reactants (LHS) | Number of atoms in products (RHS) |
| Fe | 1 | 3 |
| H | 2 | 2 |
| O | 1 | 4 |
|
Atoms of Oxygen |
||
| Atoms of Oxygen | In reactants | In products |
| Initial | 1 (in H2O ) | 4 (in Fe3O4 ) |
| To balance | 1 x 4 | 4 |
Fe + 4 H2O → Fe3O4 + H2
| Atoms of Hydrogen | ||
| Atoms of Hydrogen | In reactants | In products |
| Initial | 8 (in 4 H2O) | 2 (in H2) |
| To balance | 8 | 2 x 4 |
| Atoms of Iron | ||
| Atoms of Iron | In reactants | In products |
| Initial | 1 (in Fe) | 3 (in Fe3O4 ) |
| To balance | 1 x 3 | 3 |
3Fe + 4H2O → Fe3O4 + 4H2
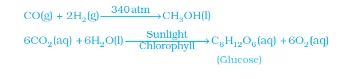
Note: The word aqueous (aq) is written if the reactant or product is present as a solution in water.
<div class="new-fform">
</div>