![]() 15 Dec 2023
15 Dec 2023
Conduction of electricity in liquids, also known as electrolytic conduction, involves the movement of charged particles within the liquid. In this process, ions, which are electrically charged atoms or molecules, facilitate the flow of electric current.
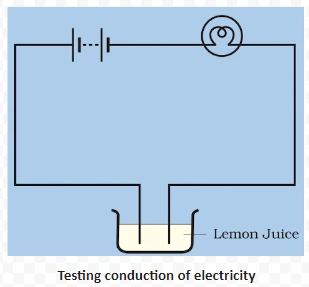
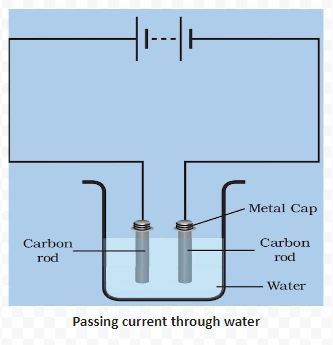
<div class="new-fform">
</div>