Acid and Base Strength: pH Scale Overview
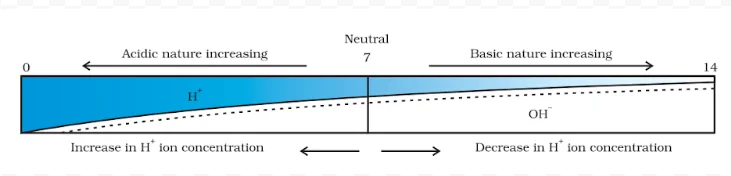
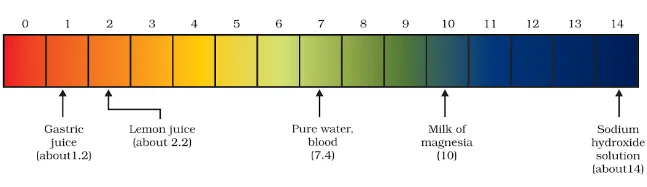
The strength of acid and base solutions is determined by their concentration of hydrogen ions (H⁺) or hydroxide ions (OH⁻), respectively. The pH scale acid and base is commonly used to express this strength. The pH scale ranges from 0 to 14, with lower values indicating acidic solutions, higher values indicating basic solutions, and 7 being neutral (pure water).
Decoding Acid and Base: Universal Indicators
Exploring the pH Scale: Acid and Base Evaluation
Acid + Base → Salt + Water (Heat is evolved)
Nature provides neutralisation options
Nettle is a herbaceous plant which grows in the wild. Its leaves have stinging hair, which cause painful stings when touched accidentally. This is due to the methanoic acid secreted by them. A traditional remedy is rubbing the area with the leaf of the dock plant, which often grows beside the nettle in the wild.
<div class="new-fform">
</div>
