![]() 14 Oct 2023
14 Oct 2023
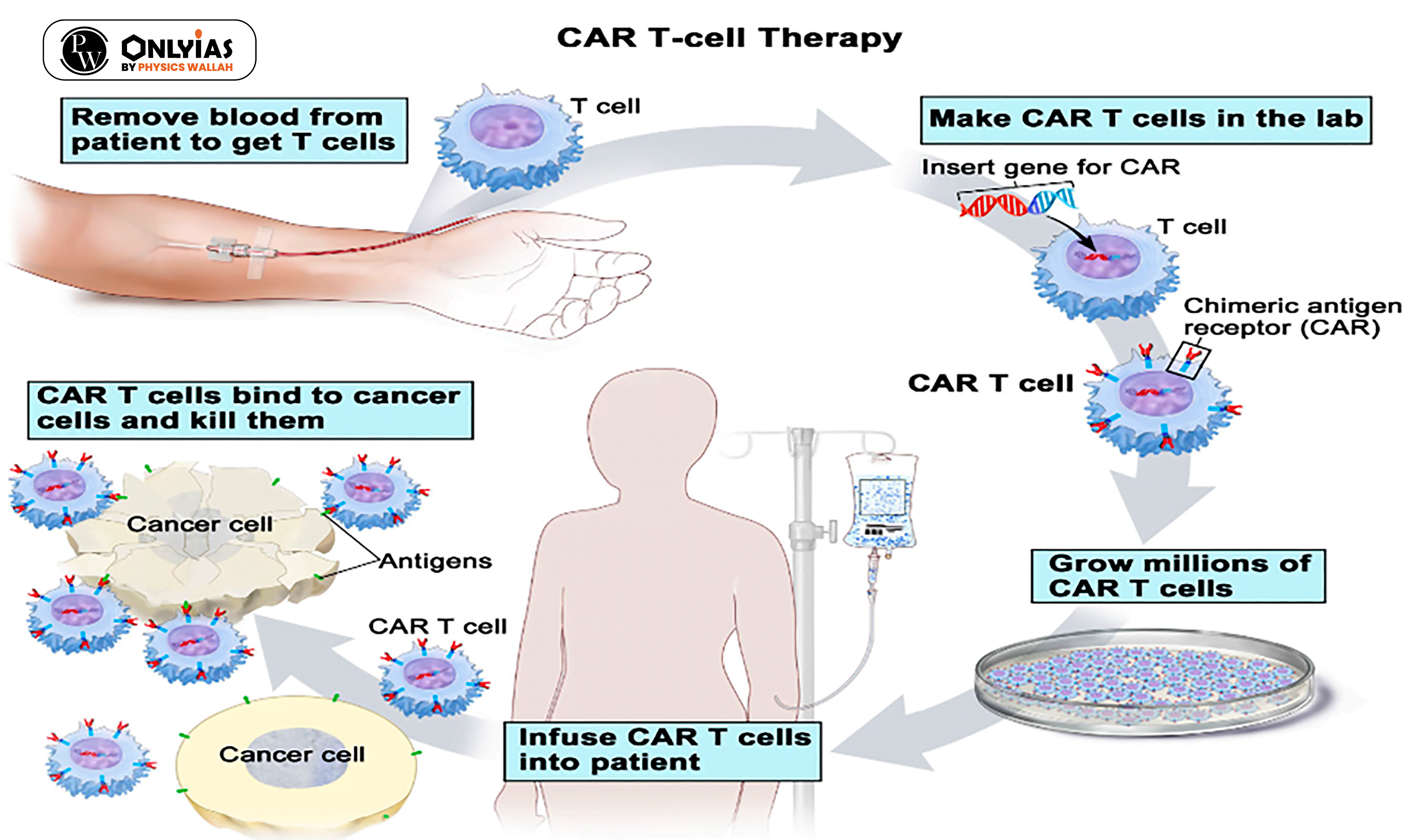
News Source: DTE
| Attempt the PY Prelims Question
Consider the following statements: (2020) 1. Genetic changes can be introduced in the cells that produce eggs or sperms of a prospective parent. 2. A person’s genome can be edited before birth at the early embryonic stage. 3. Human induced pluripotent stem cells can be injected into the embryo of a pig. Which of the statements given above is/are correct? (a) 1 only (b) 2 and 3 only (c) 2 only (d) 1, 2 and 3 Ans: (d) |
|---|
<div class="new-fform">
</div>