Context:
Recently the government directed all pharmaceutical companies in the country to implement the revised Good Manufacturing Practices (GMP), bringing their processes at par with global standards.
Directions by the Government:
- Larger companies with a turnover of over Rs 250 crore have been asked to implement the changes within six months.
- The medium and small-scale enterprises with turnover of less than Rs 250 crore have been asked to do so within a year.
About Good Manufacturing Practices (GMP):
- Quality Management: It is a set of guidelines and quality management principles which ensure pharmaceutical products, as well as other products in the food and healthcare industries, are consistently produced and controlled to meet quality standards appropriate for their intended use.
- Aspects Include: It covers all aspects of the manufacturing process, including the premises, equipment, personnel, materials, production, quality control, documentation, and storage of finished products.
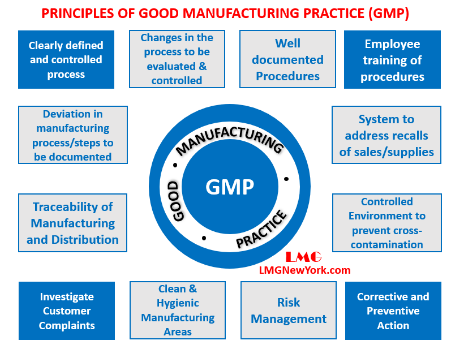 Image Credits: LMGNewYork.com
Image Credits: LMGNewYork.com
Need for the Improved Standards:
- To match up with the Global Standards: Implementation of the new norms will bring the Indian industry on par with global standards.
- Only 2,000 of the 10,500 drug manufacturing units in the country at present meet global standards, being WHO-GMP certified.
- The improved standards will ensure that pharmaceutical companies follow standard processes, quality control measures, and do not cut corners, improving the quality of medicines available in India as well as sold in the global market.
- To improve Indian Products Image: There have been a string of incidents where other countries have reported alleged contamination in India-manufactured syrups, eye-drops, and eye ointments.
- The deaths of 70 children in the Gambia, 18 children in Uzbekistan, three persons in the United States, and six deaths in Cameroon have been linked to these products.
- To rectify the Deficiencies: A risk-based inspection of 162 manufacturing units by the government found several deficiencies — incoming raw materials not being tested before use, product quality not being reviewed , absence of quality failure investigation, infrastructure deficiency to prevent cross-contamination, faulty design of manufacturing and testing areas, missing qualified professionals, and poor documentation.
- To Provide a Structure to the Draft: Implementation of the revised good manufacturing practices (GMP) as listed in the 2018 draft schedule M of the drugs and cosmetics rules.
Major Changes & Significance:
- Standard & Reliable Quality: The revised GMP guidelines focus on quality control measures, proper documentation, and IT backing to maintain quality of medicines produced.
- Review & Validation: It introduces pharmaceutical quality systems, quality risk management, product quality review and validation of equipment.
- Thorough Investigation: Carrying regular quality reviews of all its products, verify consistency of the quality and the processes, thorough investigation of any deviation or suspected defect and implementation of any preventive actions.
- Evaluation of Changes: It also suggests a change control system to evaluate all changes that may affect the production or quality of the product.
- Maintenance of Stability & Required Conditions: The companies will also be needed to mandatorily maintain the drugs in a stability chamber, set the proper temperature and humidity, and carry out an accelerated stability test as well.
- Data Safety & Security: The guidelines also state that companies should have GMP-related computerized systems, which ensure that there is no tampering of data related to the processes.
- In case sensitive data is entered manually to the system, there will be additional checks to validate the accuracy of the data. Backups would also be created to ensure there is no loss of data.
Conclusion:
The step taken is a required and desirable one. Instituting the same quality across the industry will give confidence to regulators from other countries and will improve the quality of drugs in the domestic markets.
News Source: The Indian Express
![]() 9 Aug 2023
9 Aug 2023
 Image Credits: LMGNewYork.com
Image Credits: LMGNewYork.com