Context
The Indian Institute of Technology Bombay (IIT-B) has taken initiative to create patient-centric cancer therapy in India. Their focus is on developing accessible CAR-T cell therapy.
- CDSCO approved the first CAR-T cell therapy in October 2023.
- This therapy is intended to treat relapsed or refractory B-lymphomas and B-Acute Lymphoblastic Leukemia (B-ALL), where other treatments have failed.
About Chimeric Antigen Receptor Or CAR-T Cell Therapy
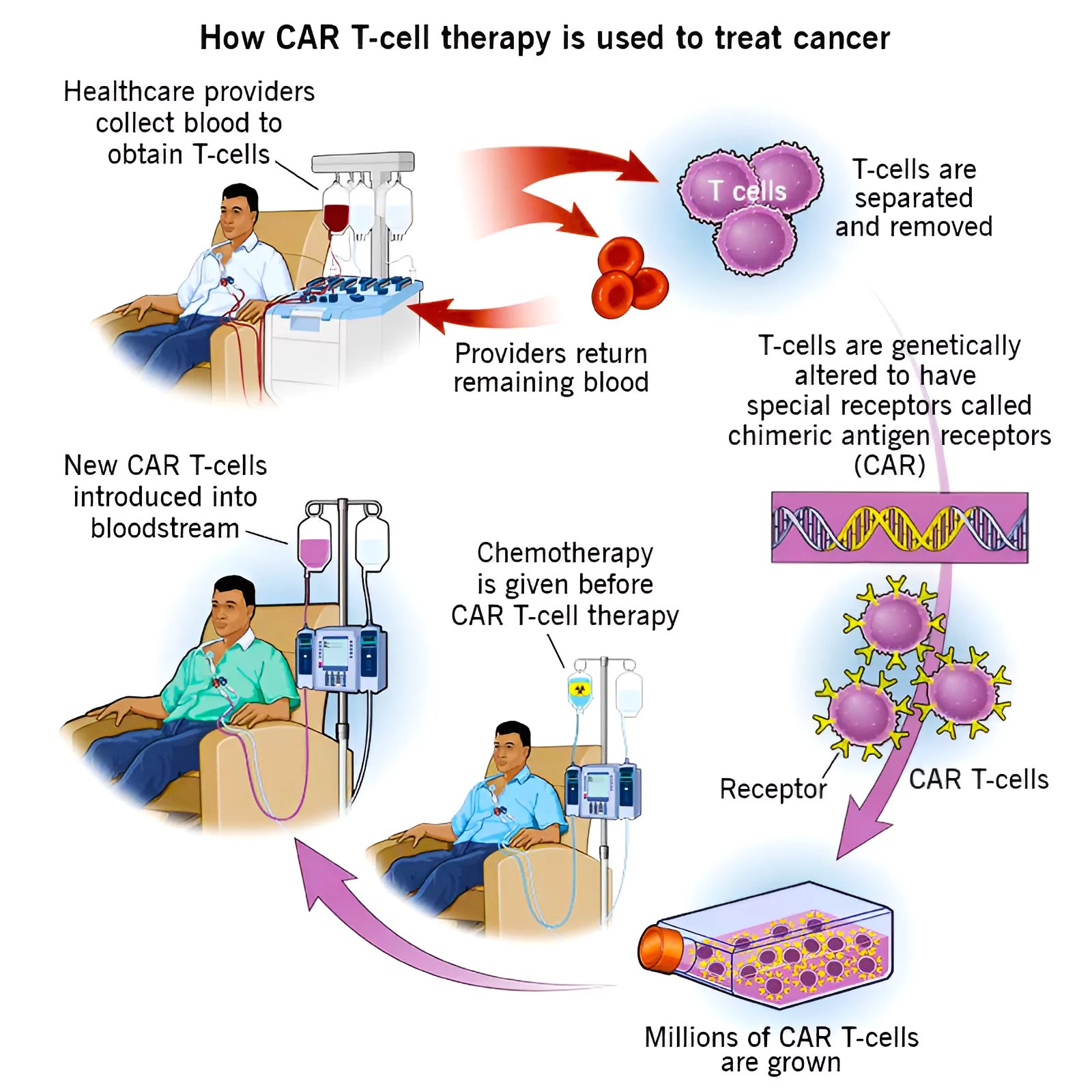
- CAR-T cell therapy is a type of cancer treatment that modifies a patient’s immune cells to target cancer cells more effectively.
- Process of CAR-T Cell Therapy:
- T cells are derived from bone marrow stem cells and are essential components of the immune system responsible for defending the body against infections.
- These cells are a type of white blood cell
- It is extracted through a procedure called leukapheresis.
- These T cells are then modified in a laboratory to express proteins called chimeric antigen receptors (CARs).
Enroll now for UPSC Online Course
-
-
-
- The CAR has different parts that help it recognize cancer cell antigens and activate the immune system.
 Each CAR stretches across the cell membrane, with parts outside and inside the cell.
Each CAR stretches across the cell membrane, with parts outside and inside the cell.
- Outside, there are fragments of antibodies made in the lab.
- It is chosen because they stick well to the target.
- Inside, there are two components that send signals when the receptor meets an antigen.
- The gene that makes the CAR is artificially created in the laboratory.
- They then use a carrier called a vector to deliver this gene into the patient’s T-cells.
- Viral vectors, like lentiviral vectors, are often used in this process.
- The modified T cells are then multiplied in the lab before being infused back into the patient’s body.
- The patient usually undergoes chemotherapy before receiving the CAR-T cells.
FDA Approval:
- The FDA has given the green light to six CAR-T cell therapies so far.
- Four of these therapies are aimed at a protein called CD19 found on the surface of leukemia and lymphoma cells.
- NexCAR19: Similarities and Differences:
- NexCAR19 shares similarities with these therapies as it also targets CD19.
- NexCAR19 is a CAR-T therapy developed in India.
- Difference between US-developed therapies and NexCAR19.
- In the US, CAR-T cell therapies use antibody fragments from mice.
- NexCAR19, on the other hand, has human proteins mixed with the mouse antibodies, making it more ‘human-like.’
- Objective: This modification aims to enhance the therapy’s safety profile and effectiveness.
Applications of CAR-T cell therapy
It is mainly used for certain types of blood cancers:
- B-cell acute lymphoblastic leukemia (ALL).
- Diffuse large B-cell lymphoma.
- Follicular lymphoma.
- High-grade B-cell lymphoma.
- Mantle cell lymphoma
- Multiple myeloma.
- Primary mediastinal large B-cell lymphoma.
Risks of CAR-T cell therapy
- Cytokine Release Syndrome (CRS): This is the most common side effect and involves an intense inflammatory response.
- This can cause Fever, Chills, Fatigue, Muscle aches, Nausea and vomiting, Difficulty breathing, Low blood pressure
- In severe cases, it can be life-threatening.
- Neurotoxicity: It can affect the nervous system and cause Confusion, Tremors, Seizures, Difficulty speaking. Loss of coordination.
- Increased Risk of Infections: CAR-T therapy weakens the immune system, making it harder for the body to fight off infections. This is especially concerning because many patients receiving CAR-T therapy are already battling cancer.
- Low Blood Cell Counts: Treatment can decrease the production of important blood cells, leading to Fatigue, Increased risk of bleeding, Higher chance of infection.
Enroll now for UPSC Online Classes
Challenges Before CAR-T Therapy Implementation in India
- Limited access to primary healthcare: Primary healthcare facilities are a challenge in many parts of India.
- This can be a barrier for patients considering CAR-T therapy, as this therapy requires close monitoring and management by specialists throughout the process.
- Concentration of cancer treatment facilities: Cancer treatment facilities, especially those equipped for advanced therapies like CAR-T, are concentrated in metropolitan areas.
- This can create a disparity for patients in remote locations who may not be able to easily access the therapy or the required follow-up care.
- Infrastructure for managing side effects: CAR-T therapy has various side effects, particularly cytokine release syndrome (CRS). Therefore, it requires intensive care support.
- However, the limited availability of such facilities in some areas could be a challenge.
- Immunocompromised patients: CAR-T therapy itself can leave patients immunocompromised, making them more susceptible to infections. India’s healthcare system may not be universally equipped to handle such cases effectively, especially in areas with limited resources.
- High Cost: Even though NexCAR19 is a more affordable option compared to similar treatments abroad, it remains expensive for many Indian patients, ranging from ₹40 to 45 lakh.
- There are multiple aspects that contribute to the high cost of NexCAR19 production. These include Labor expenses, Logistics, Materials, Facility costs, Marketing, distribution, and intellectual property development.
Also Read: Cancer Prevalence In India
![]() 12 Apr 2024
12 Apr 2024
 Each CAR stretches across the cell membrane, with parts outside and inside the cell.
Each CAR stretches across the cell membrane, with parts outside and inside the cell.