Context: This article is based on an Editorial “Game-changer: On approval for gene therapies to treat sickle cell disease and beta thalassemia” which was published in the Hindu. Recently, the US Food and Drug Administration (FDA) has approved two gene therapies — Casgevy and Lyfgenia — to treat sickle cell disease in patients over 12.
- Earlier, the UK drug regulator approved Casgevy to treat people above 12 with sickle cell disease and beta thalassemia.
| Relevancy for Prelims: Gene, CRISPR-Cas9, Genome Editing, Casgevy and Lyfgenia.
Relevancy for Mains: Gene Therapies to Treat Sickle Cell Disease: Significance and Concerns. |
About Sickle Cell Disease (SCD)
- Blood Disorder: It is an inherited blood disorder marked by defective hemoglobin.
- Impact on Oxygen Carrying Ability: The SCD inhibits the ability of hemoglobin in red blood cells to carry oxygen.
- Threat: Sickle cells tend to stick together, blocking small blood vessels causing painful and damaging complications.
About Gene and Genome Editing
- Unit of Hereditary: A gene is the structural and functional unit of heredity, which are made up of Deoxyribonucleic acid (DNA).
- Some genes act as instructions to make molecules called proteins.
- Gene Editing: It is a type of genetic engineering in which DNA is inserted, deleted, or altered in the genome of a living organism.
- It targets the insertions of required genes to site specific locations.
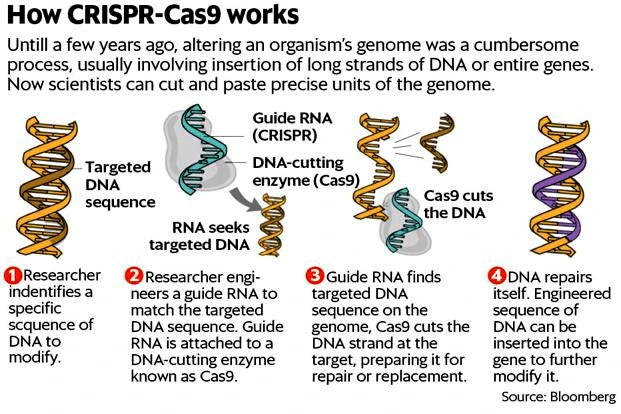
About CRISPR-Cas9
- CRISPR: It is considered as the most precise, cost-effective and quickest way to edit genes.
- CRISPR-Cas9: It is a unique technology that enables geneticists and medical researchers to edit the required genome by removing, adding or altering sections of the DNA sequence.
Gene Therapies to Treat Sickle Cell: Lyfgenia
- Process Involved: It uses a disabled lentivirus as a vector to introduce into the blood stem cells a new gene for hemoglobin same as the healthy gene.
- Achievement: In clinical trials, 30 of 32 sickle cell disease patients did not suffer from severe blocked blood flow caused by sickle cells, while 28 of 32 patients did not experience any blocked blood flow events six to 18 months post-infusion.
Gene Therapies to Treat Sickle Cell: Casgevy
- Process Involved: It uses the gene-editing tool of CRISPR-Cas9 to disable a particular gene (BCL11A) that turns off fetal hemoglobin production in blood stem cells.
- By disabling the BCL11A gene, the produced fetal hemoglobin does not have the abnormalities of adult hemoglobin, hence, helps to treat patients with sickle-cell disease or beta thalassaemia.
- Achievement: In clinical trials, 28 of 29 sickle-cell disease patients who received Casgevy gene therapy were relieved from the disease for a year.
- For beta thalassaemia, 39 of 42 patients did not require blood transfusion for one year, and in the remaining three the need for blood transfusion reduced by more than 70%.
Read more about Casgevy Therapy – A Gene Therapy for Sickle Cell Disease, here.
Gene Therapy: Significance
- Use of CRISPR-Cas9 Tool: These mark the beginning of gene therapy using the CRISPR-Cas9 tool to treat diseases that could otherwise be cured only through bone marrow transplantation.
- No Dependency on Donor: As both gene therapies use patients’ own blood cells for gene editing. These treatments do not rely on matching bone marrow donors.
What concerns surround the use of gene therapies?
- Expensive: These treatments would be extremely expensive.
- Limited Availability of Treatment: Similar to bone marrow transplantation, these facilities would be available only in certain hospitals.
- Observation only through Less Clinical Trials: The available data is not a guarantee of safety and efficacy to the entire World.
- Side Effects: There is a possibility of unintended genetic modifications and their resultant side effects by using the CRISPR–Cas9 tool.
Conclusion:
Gene therapies offers new hope for those with sickle cell disease. The time has come to widen the reach of clinical trials and take actions on higher penetration of therapy to most of the treatment places in an cost-effective manner.
| Prelims Question (2019)
What is Cas9 protein that is often mentioned in news?
(a) A molecular scissors used in targeted gene editing
(b) A biosensor used in the accurate detection of pathogens in patients
(c) A gene that makes plants pest-resistant
(d) A herbicidal substance synthesized in genetically modified crops
Ans: (a) |
| Mains Question (2017): Stem cell therapy is gaining popularity in India to treat a wide variety of medical conditions including leukaemia, Thalassemia, damaged cornea and several burns. Describe briefly what stem cell therapy is and what advantages it has over other treatments? |
![]() 13 Dec 2023
13 Dec 2023