Ozone Layer Depletion is covered here. When ozone molecules come into contact with chlorine and bromine atoms in the atmosphere, they break down, causing this phenomenon.

Ozone layer depletion refers to the thinning of the ozone layer in the Earth’s stratosphere, particularly in the region known as the ozone hole. The ozone layer is a crucial part of the Earth’s atmosphere as it absorbs the majority of the Sun’s harmful ultraviolet (UV) radiation. This radiation has the potential to cause various health issues in humans, damage to ecosystems, and harm to animals.
| Ozone Layer Depletion | |
|---|---|
| Meaning | The breakdown of ozone molecules in the stratosphere due to the presence of ozone-depleting substances (ODS). |
| Effects | Increased UV radiation reaching Earth’s surface, Damage to aquatic food chains, Ozone hole formation (e.g., over Antarctica), Weakening of the immune system, Impact on weather and climate patterns and Economic costs |
| Prevention & Mitigation | Montreal Protocol |
World Ozone Day is celebrated annually on September 16 to increase awareness of the vital role the ozone layer plays in preserving life on Earth. The Vienna Convention for the Protection of the Ozone Layer, which was signed forty years ago, committed governments to taking the necessary precautions to shield the earth and its people from dangerous UV radiation, which could breach the ozone layer. Chlorofluorocarbons, which are synthetic compounds used in refrigeration, aerosol sprays, and foam creation, began to be phased out when the Montreal Protocol was approved based on more scientific data, putting the ozone layer on the path to recovery.
This year, on International Day for the Preservation of the Ozone Layer, we celebrate this historic achievement and look forward to another four decades of activity. Monitoring ozone and UV radiation levels, as well as ozone-depleting compounds and other chemicals, such as hydrofluorocarbons (greenhouse gases), which are being phased out under the Kigali Amendment, depends on the Vienna Convention and the Montreal Protocol. The concept of moving from science to global action has been reflected in the ozone conventions. And for many years to come, they will continue to do so.
The ozone layer refers to a section in the Earth’s stratosphere with elevated levels of ozone, safeguarding the planet from the detrimental ultraviolet rays emitted by the sun.
Also Read:
The primary cause of ozone layer depletion is the release of certain man-made chemicals into the atmosphere, specifically chlorofluorocarbons (CFCs), halons, and other ozone-depleting substances. These chemicals were commonly used in refrigerants, aerosol propellants, foam-blowing agents, and other industrial processes.
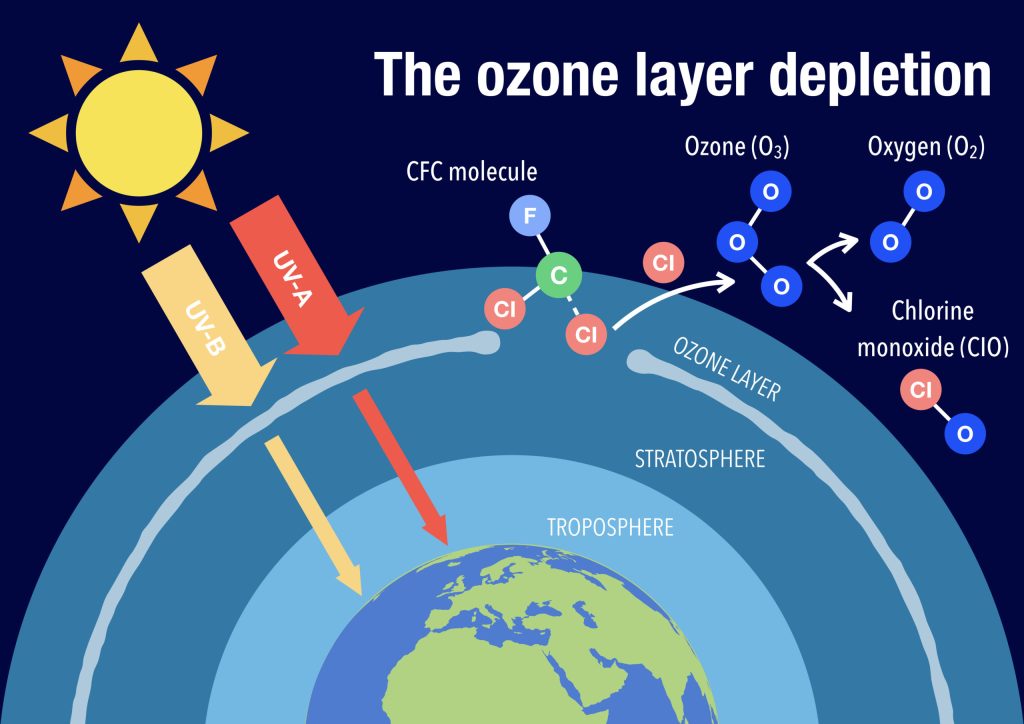
When these chemicals are released into the atmosphere, they eventually reach the stratosphere, where they are broken down by the Sun’s ultraviolet radiation. This process releases chlorine and bromine atoms, which then catalytically destroy ozone molecules.
The discovery of the Antarctic ozone hole in the 1980s raised global concern about the harmful effects of ozone layer depletion. The depletion of the ozone layer allows more UV radiation to reach the Earth’s surface, which can lead to increased rates of skin cancer, cataracts, and other health problems. It can also affect terrestrial and marine ecosystems, including phytoplankton and other aquatic organisms at the base of the food chain.
Situated primarily in the lower part of Earth’s atmosphere, the ozone layer plays a critical role. It absorbs a substantial portion, around 97-99%, of the harmful ultraviolet rays emitted by the sun. This protective function prevents these ultraviolet radiations from causing damage to life on our planet. Without the ozone layer, the consequences could be dire, leading to an increased prevalence of skin diseases and weakened immune systems among millions of people.
Ozone layer depletion is primarily caused by human activities and certain natural processes. Here are the main causes:
Chlorofluorocarbons (CFCs): CFCs are synthetic compounds that were commonly used in aerosol sprays, refrigerants, and foam-blowing agents. When released into the atmosphere, they eventually reach the stratosphere, where they break down due to the intense ultraviolet (UV) radiation. This breakdown releases chlorine atoms, which then catalytically destroy ozone molecules.
Halons: Halons are similar to CFCs and are often used in fire extinguishers. They release bromine and chlorine when they break down, both of which are ozone-depleting substances.
Carbon Tetrachloride: This chemical compound, once used in the production of CFCs, can release chlorine into the stratosphere when it breaks down.
Methyl Chloroform: Like CFCs, methyl chloroform releases chlorine when it breaks down, contributing to ozone depletion.
Hydrochlorofluorocarbons (HCFCs): While HCFCs were developed as a less harmful replacement for CFCs, they still have some ozone-depleting potential. They are being phased out in favor of more ozone-friendly alternatives.
Nitrous Oxide: This is a natural source of ozone depletion. Agricultural and industrial activities release nitrous oxide, which can reach the stratosphere and break down ozone molecules.
Natural Processes: Volcanic eruptions and forest fires release gases that can contribute to ozone depletion. However, these natural processes have a relatively small impact compared to human-made ozone-depleting substances.
Solar Variability: Changes in solar radiation can influence ozone levels, but this is a minor factor compared to human-induced ozone depletion.
Ozone-depleting substances (ODS) are chemical compounds that, when released into the atmosphere, have the potential to deplete the ozone layer in the Earth’s stratosphere. These substances contain chlorine, fluorine, bromine, carbon, or hydrogen atoms that can catalytically destroy ozone molecules when they reach the stratosphere. ODS include:
Chlorofluorocarbons (CFCs): CFCs are synthetic compounds that were widely used in refrigeration, air conditioning, aerosol propellants, and foam-blowing agents before their harmful effects on the ozone layer were discovered. They are stable in the lower atmosphere but release chlorine atoms when they break down in the stratosphere.
Halons: Halons are similar to CFCs and contain bromine and chlorine. They were commonly used in fire extinguishers and are potent ozone-depleting substances.
Carbon Tetrachloride: This chemical compound was used in the production of CFCs and releases chlorine into the stratosphere when it breaks down.
Methyl Chloroform: Like CFCs and carbon tetrachloride, methyl chloroform releases chlorine when it breaks down, contributing to ozone depletion.
Hydrochlorofluorocarbons (HCFCs): HCFCs were developed as transitional substitutes for CFCs because they have lower ozone-depleting potential. However, they are still considered ODS and are being phased out in favor of more ozone-friendly alternatives.
Hydrobromofluorocarbons (HBFCs): Similar to HCFCs, HBFCs contain bromine, and they were used in some industrial applications and foam products.
Hydrochlorobromofluorocarbons (HCBFCs): These compounds combine chlorine, bromine, and fluorine atoms and were used in some specialized applications.
Nitrous Oxide (N2O): Although primarily a greenhouse gas, nitrous oxide can contribute to ozone depletion when it reaches the stratosphere. It releases nitrogen oxides that can react with ozone.
Chlorobromomethane: This is used in some fire extinguishers and also contains chlorine and bromine, making it an ozone-depleting substance.
The depletion of the ozone layer has significant and far-reaching effects on the environment, human health, and ecosystems. Here are some of the primary effects:
Increased Ultraviolet (UV) Radiation: Ozone in the stratosphere absorbs and blocks a significant portion of harmful ultraviolet (UV) radiation from the sun. As the ozone layer thins, more UV radiation reaches the Earth’s surface. Increased UV exposure can lead to various health problems, including:
Harm to Marine Life: UV radiation can penetrate water and harm marine ecosystems. It can damage phytoplankton, which forms the base of the ocean food chain, and affect coral reefs. This disruption can have cascading effects on marine life.
Impact on Terrestrial Ecosystems: Increased UV radiation can damage plant DNA, leading to reduced crop yields, lower agricultural productivity, and damage to natural vegetation.
Disruption of Aquatic Food Chains: UV radiation can harm aquatic organisms, including fish and amphibians, disrupting aquatic food chains and ecosystems.
Ozone Hole: In some regions, particularly over Antarctica, the ozone layer has developed a seasonal “hole” due to severe ozone depletion. This hole allows even more UV radiation to reach the surface, causing heightened concerns for those living in or near affected areas.
Human Health Impacts: Beyond skin cancer and eye damage, increased UV radiation can cause sunburn, premature aging of the skin, and exacerbate conditions like lupus and polymorphous light eruption.
Environmental Changes: Ozone depletion can alter atmospheric circulation patterns, potentially affecting weather and climate in certain regions.
Economic Costs: Increased UV radiation and its effects on agriculture, tourism, and healthcare systems can lead to substantial economic costs for affected countries.
Governments across the globe have initiated various programs to combat the alarming destruction of the ozone layer, a pressing global concern. However, individual actions also play a pivotal role in preventing further ozone layer depletion. Here are some ideas that can contribute to tackling this issue on a global scale:
Reduce Ozone-Depleting Chemicals: Minimize the use of ozone-depleting chemicals in everyday life. Opt for alternative fire extinguishers that don’t contain halons and refrain from using CFCs in refrigerators and air conditioners, among other appliances.
Limit Automobile Usage: Automobiles are significant contributors to greenhouse gas emissions, which not only worsen ozone depletion but also contribute to global warming. Whenever possible, consider alternatives like public transportation, carpooling, biking, or walking to reduce your carbon footprint.
Promote Green Cleaning Supplies: Conventional cleaning products often contain chemicals that release chlorine and bromine into the atmosphere, negatively affecting the ozone layer. Choose eco-friendly, natural cleaning alternatives to help preserve the environment.
Ban Nitrous Oxide Use: Governments should consider implementing bans on the use of hazardous nitrous oxide, a substance that harms the ozone layer. Raising public awareness about the negative consequences of nitrous oxide and its sources can also reduce its personal use.
The Montreal Protocol is an international treaty designed to protect the Earth’s ozone layer by phasing out the production and consumption of ozone-depleting substances (ODS). It is widely regarded as one of the most successful international environmental agreements. Here are the key aspects of the Montreal Protocol:
Background:
Key Provisions:
Achievements:
Amendments and Adjustments:
The primary factors behind ozone depletion and the formation of the ozone hole are synthetic chemicals, particularly manufactured halocarbon compounds used in refrigerants, solvents, propellants, and foam-blowing agents. These compounds, known as ozone-depleting substances (ODS), encompass chlorofluorocarbons (CFCs), HCFCs, and halons.
Ozone layer depletion refers to the gradual reduction of the ozone layer in the upper atmosphere. This phenomenon occurs when certain chemical compounds, upon exposure to intense ultraviolet light, release chlorine and bromine, contributing to the reduction of ozone levels.
The ozone layer plays a crucial role in protecting us from the sun's detrimental ultraviolet rays. However, when the ozone layer gets depleted, humans become vulnerable to these harmful rays, leading to the onset of skin diseases, cataracts, cancer, compromised immune systems, and more.
The depletion of the ozone layer results in heightened levels of UVB reaching the Earth's surface. Research conducted in laboratories and through epidemiological studies highlights that UVB exposure contributes to non-melanoma skin cancer and significantly influences the progression of malignant melanoma.
These substances play a role in causing ozone depletion and are referred to as ozone-depleting substances (ODS). ODS encompass chlorofluorocarbons (CFCs), hydrochlorofluorocarbons (HCFCs), halons, methyl bromide, carbon tetrachloride, hydrobromofluorocarbons, chlorobromomethane, and methyl chloroform.

<div class="new-fform">
</div>