The Central Licensing Authority (CLA) has recently specified six countries, United States, United Kingdom, Japan, Australia, Canada, and the European Union, under Rule 101 of the New Drugs and Clinical Trials Rules (NDCTR), 2019.
Benefits for Patients and the Industry
- Accelerated Drug Availability: Faster approvals for life-saving drugs for rare diseases and pandemic situations. Reduced waiting periods for patients with critical conditions.
Five categories of new drugs allowed for Waiver
- Orphan drugs for rare diseases
- Gene and cellular therapy products
- Drugs for pandemic situations
- Drugs for special defense purposes
- Drugs offering significant therapeutic advancements
|
Enroll now for UPSC Online Course
- Enhanced Industry Response: Simplified regulatory pathways for new drug launches. Better preparedness to respond to public health emergencies.
- Patient Advocacy Perspectives: Advocacy groups see fast-track approvals as a significant step forward. The move addresses challenges in accessing treatments for rare diseases.
Risks of Waiving Local Trials
- Patient Safety and Genetic Diversity: India’s diverse genetic makeup necessitates local trials to assess drug safety and efficacy. Waiving trials risks unanticipated adverse effects due to genetic variability.
- Countries like Japan and China mandate local testing or foreign data analysis for ethnic sensitivity, highlighting its importance.
- Gene and Cellular Therapies: Long-term risks of therapies like CAR-T (which manipulate immune cells) are not fully understood. Local trials are crucial to account for genetic responses unique to India.
- Impact on Research and Development (R&D): The waiver could weaken incentives for localized research.India’s pharmaceutical innovation and research infrastructure, including contract research organizations (CROs), may face reduced investments.
- Advantages for Multinational Corporations (MNCs): MNCs may benefit disproportionately, with faster market entry and fewer regulatory hurdles.
- Concerns from Patient Groups: Despite the benefits, patient groups worry about limited access to costly drugs that were earlier available through clinical trials. Inadequate safety validation for new therapies, particularly those with significant advancements.
Regulatory Assurances
The Drugs Controller General of India (DCGI) has clarified that:
- Waivers will apply only to drugs approved and marketed in the specified countries.
- No major unexpected serious adverse events must have been reported in these countries for the waiver to be granted.
Conclusion
The local clinical trial waiver represents a critical regulatory shift in India’s drug approval framework. While it promises faster access to essential medications, it necessitates a balanced approach to address safety concerns, promote localized research, and ensure equitable patient benefits
Check Out UPSC CSE Books From PW Store
What is a Clinical Trial?
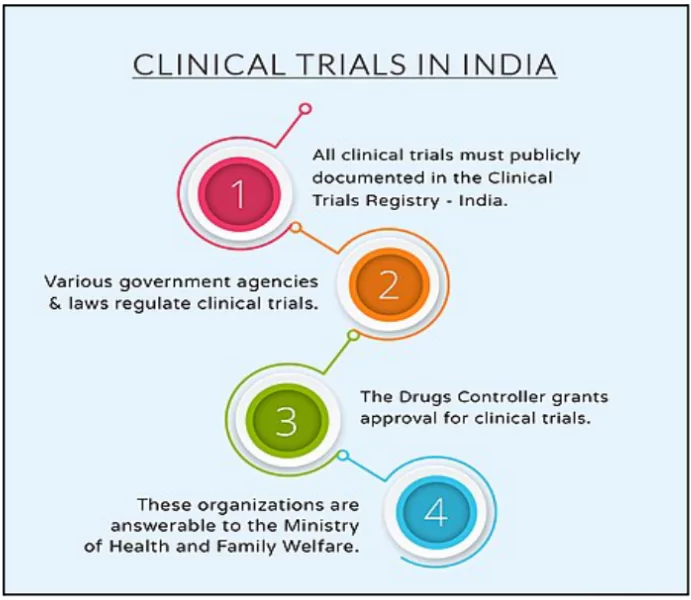
- A systematic study to evaluate the safety and effectiveness of a new drug or treatment in humans.
- Involves multiple phases, starting with small groups and gradually increasing the number of participants.
- Essential for ensuring the safety and efficacy of new medications before they are approved for widespread use.
- Regulatory Mechanism in India: Drugs and Cosmetics Act, 1940, Medical Council of India Act, 1956 and Central Council for Indian Medicine Act, 1970.
Clinical Trials Registry of India (CTRI)
- Established in July 2007, CTRI is a publicly accessible online platform hosted by the Indian Council of Medical Research’s (ICMR) National Institute of Medical Statistics.
- Initially voluntary, registration of clinical trials became mandatory in June 2009 as per the Drug Controller General of India (DCGI).
- The platform ensures transparency and accountability for studies involving human participants, including drugs, surgical procedures, medical devices, and other interventions.
- Sponsors must publicly declare trials, detail investigators, obtain DCGI and ethics committee approvals, and outline participant selection criteria.
- CTRI is part of the International Clinical Trials Registry Platform (ICTRP) and is recognized as a primary registry by the World Health Organization (WHO).
|
![]() 27 Dec 2024
27 Dec 2024
