Introduction: Exploring the Role of Solar Radiation
This article explores Earth’s atmosphere, including its composition, focusing on gasses like nitrogen, oxygen, and carbon dioxide. It also discusses the atmosphere’s structure with layers such as the troposphere and stratosphere, highlighting their role in climate regulation.
What is the Atmosphere? – Solar Radiation and the Atmosphere
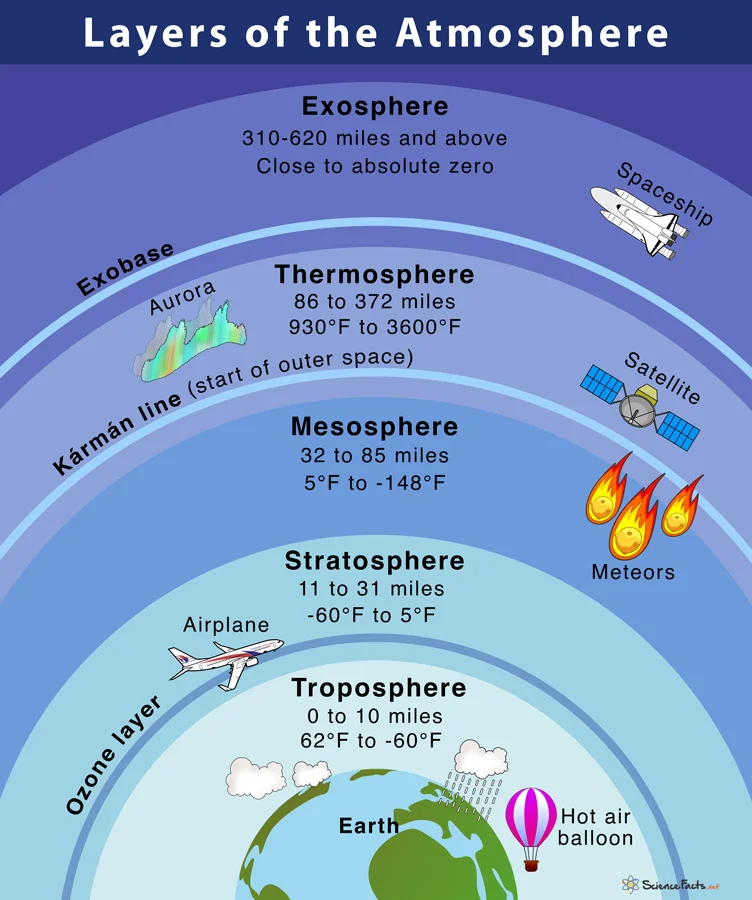
- The Earth’s Protective Shield
-
- The Earth’s envelope of gasses is known as the atmosphere.
- It acts as a crucial boundary separating outer space from the biosphere.
- A Dynamic Mix of Gases
-
- The atmosphere is a dynamic collection of gases that continually move and change.
- It comprises multiple layers around the Earth, categorized by their composition and temperature.
- The Evolution of Atmospheric Gases
-
- The gasses in our present atmosphere are not remnants from the Earth’s early formation.
| Eon/Period | Characteristics of the Atmosphere |
| Hadean Eon
(4,540 – 4,000 mya) |
|
|
|
|
|
|
|
| Archean Eon
(4,000 mya – 2,500 mya) |
|
|
|
|
|
|
|
| Proterozoic Eon
(2,500 mya – 541 mya) |
|
|
|
| Phanerozoic Eon
(541 mya to Present) |
|
|
|
|
|
|
- The Lifesaver for Earth’s Ecosystem
-
- The atmosphere is a vital component of the biospheric ecosystem.
- It is indispensable for supporting life on Earth; without it, our planet would resemble the lifeless moon.
- Protection from Solar Radiation
-
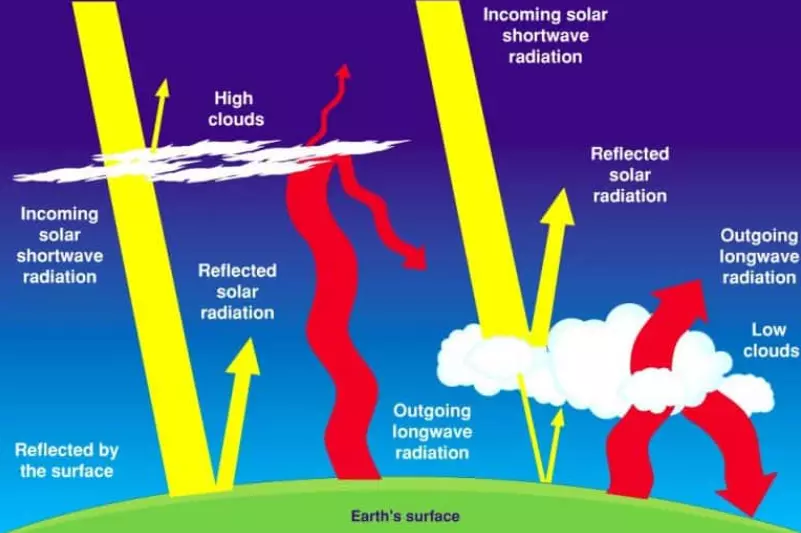
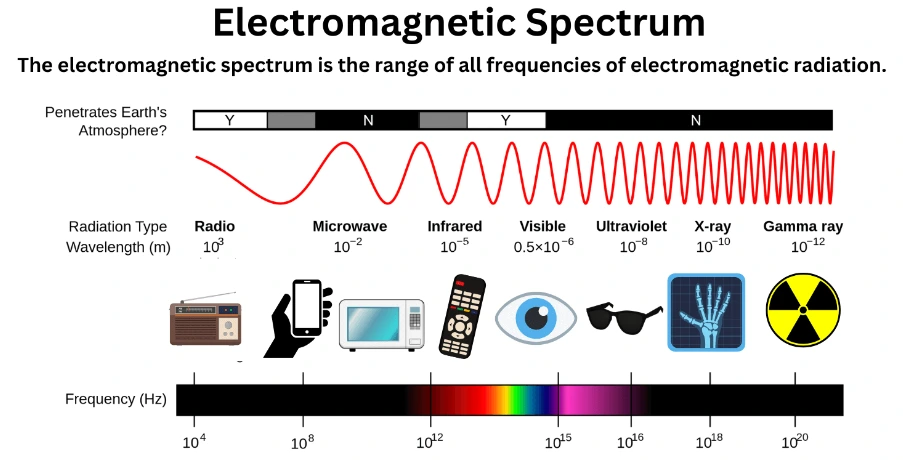
- The atmosphere shields the Earth from harmful solar radiation.
- It acts as a greenhouse by permitting short-wave radiation from the Sun while trapping long-wave terrestrial radiation from the Earth’s surface.
- Regulating Solar Radiation
-
- All life forms require specific temperature and solar radiation frequency ranges for their biophysical processes.
- The atmosphere selectively absorbs certain solar radiation frequencies and allows others, effectively regulating the entry of solar radiation.
- Temperature Control
-
- The atmosphere helps maintain a stable temperature range over the Earth’s surface.
- Without it, extreme temperature fluctuations between day and night would occur.
- Protection from Meteors
-
- The atmosphere plays a role in safeguarding the Earth from extraterrestrial objects such as meteors.
- Meteors burn up while passing through the atmosphere, particularly in the mesosphere, due to friction.
Composition of Earth’s Atmosphere: Its Role in Solar Radiation
- Gaseous Components
-
- The gasses in Earth’s atmosphere are composed of uncharged particles.
- Except for noble gasses, most gas-phase atoms share electrons through chemical bonds to attain a more stable filled-shell configuration.
- A Mix of Gases and Molecules
-
- Earth’s atmosphere comprises a combination of noble gas atoms and various types of molecules.
- It can be divided into three main components: gases, vapor, and particulates.
Composition and Significance of Atmospheric Gases
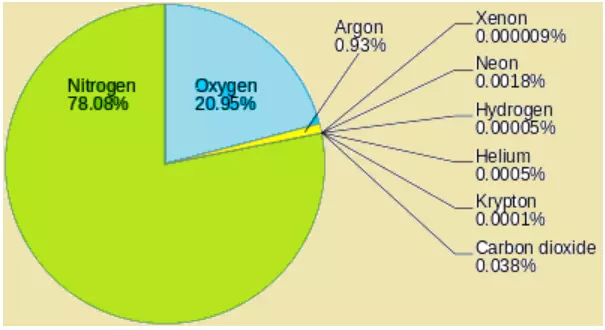
| Gas | Percentage by Volume |
|---|---|
| Nitrogen (N2) | 78.08 |
| Oxygen (O2) | 20.95 |
| Argon (Ar) | 0.93 |
| Carbon Dioxide (CO2) | 0.036 |
| Neon (Ne) | 0.002 |
| Helium (He) | 0.0005 |
| Krypton (Kr) | 0.001 |
| Methane (CH4) | 0.000179 |
| Xenon (Xe) | 0.00009 |
| Hydrogen (H2) | 0.00005 |
- Nitrogen and oxygen constitute nearly 99% of the clean, dry air.
- Oxygen, despite making up only 21% of the atmosphere, is crucial for all living organisms and combustion.Nitrogen, at 78%, dilutes oxygen and plays a role in oxidation.
- Carbon dioxide, though a small fraction (0.038%), is a product of combustion and impacts climate due to its heat-absorbing properties.
- Increased carbon dioxide, resulting from burning fossil fuels, can lead to significant temperature rises and drastic climatic changes.
- Argon, making up about 0.93%, is another significant gas.
- Ozone, denoted as O3, is a unique type of oxygen molecule comprised of three oxygen atoms. It makes up a minuscule fraction, less than 0.00005%, of the Earth’s atmospheric volume and is distributed unevenly.
- Ozone is primarily produced at higher altitudes through the interaction of oxygen molecules (O2) with ultraviolet (UV) light and is subsequently transported downward.
- The most significant concentrations of ozone are typically situated between altitudes of 20 to 30 kilometers in the lower stratosphere. At this specific atmospheric layer, ozone plays a pivotal role in shielding the Earth from the harmful ultraviolet radiation emitted by the sun.
- Other gases like neon, helium, hydrogen, xenon, krypton, and methane are present in negligible quantities.
- Potato chip packets are infused with nitrogen to inhibit the onset of rancidity, which occurs due to the oxidation of the oils and fats contained within the chips.
- Electric bulbs are filled with non-reactive gases like argon and nitrogen to shield the tungsten filament from exposure to oxygen, thus preventing it from burning.
Atmospheric Water Vapor: Sources, Distribution, and Impacts on Weather and Climate
- Atmospheric water vapor content ranges from 0 to 5% by volume.
- It arises from the evaporation of water from various sources such as seas, oceans, lakes, vegetation, and soil.
- Vapor content decreases with decreasing temperature from the equator to the poles.
- Most atmospheric vapor, over 90%, exists below 5 km altitude.
- Moisture in the atmosphere leads to condensation and various forms of precipitation, like clouds, fog, dew, rain, frost, hail, and snowfall.
- Water vapor allows shortwave solar radiation to pass through but absorbs longwave terrestrial radiation, contributing to heating the Earth’s surface and lower atmosphere.
Particulate Matter
- Solid particles in the atmosphere consist of sand, pollen, small organisms, soot, ocean salts, and even meteor fragments.
- These particles play a role in absorbing, reflecting, and scattering solar radiation, creating the colors seen at sunrise and sunset.
- The blue appearance of the sky is due to selective scattering of solar radiation by dust particles.
- Salt particles become hygroscopic nuclei, aiding in the formation of water droplets, clouds, and various types of condensation and precipitation.
Hygroscopic Nuclei: A microscopic particle (e.g., sulfur dioxide, salt, dust, or smoke) in the free air, onto which water vapor may condense to form droplets.
Prelims PYQs
- Consider the following statements: (2018)
- The Earth’s magnetic field has reversed every few hundred thousand years.
- When the Earth was created more than 4000 million years ago, there was 54% oxygen and no carbon dioxide.
- When living organisms originated, they modified the early atmosphere of the Earth.
Which of the statements given above is/are correct?
(a) 1 only
(b) 2 and 3 only
(c) 1 and 3 only
(d) 1, 2 and 3
Ans. C
- Consider the following: (2013)
- Electromagnetic radiation
- Geothermal energy
- Gravitational force
- Plate movements
- Rotation of the earth
- Revolution of the earth
Which of the above are responsible for bringing dynamic changes on the surface of the earth?
(a) 1, 2, 3 and 4 only
(b) 1, 3, 5 and 6 only
(c) 2, 4, 5 and 6 only
(d) 1, 2, 3, 4, 5 and 6
Ans. d
Mains Practice Question:
- Describe the various roles of water vapor in the atmosphere, including its impact on weather patterns, precipitation, and the greenhouse effect. (150 words, 10 marks)
- Explain the concept of hygroscopic nuclei in the atmosphere and how they contribute to the formation of clouds and precipitation. Provide examples of their significance in atmospheric processes. (150 words, 10 marks)
Mains PYQs
Q. How far do you agree that the behaviour of the Indian monsoon has been changing due to humanising landscapes? Discuss. (2015)

 GS Foundation
GS Foundation Optional Course
Optional Course Combo Courses
Combo Courses Degree Program
Degree Program